CCK-4
 | |
| IUPAC ime | |
|---|---|
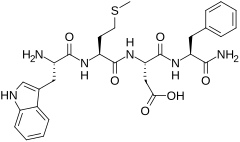
(3S)-3-[(2S)-2-amino-3-amino-3-fenilpropanamido]-3-{[(1S)-1-{[(1S)-1-karboksi -2-(indol-3-il)etil]karbamoil}-3-(metilsulfanil)propil]karbamoil}propanoinska kiselina | |
| Klinički podaci | |
| Način primene | IV |
| Farmakokinetički podaci | |
| Bioraspoloživost | 100% |
| Metabolizam | proteaze plazme |
| Poluvreme eliminacije | 13 minuta |
| Izlučivanje | N/A |
| Identifikatori | |
| CAS broj | 1947-37-1 |
| Hemijski podaci | |
| Formula | C29H35N5O7S |
| Molarna masa | 597.681 g/mol |
| |
CCK-4 (holecistokininski tetrapeptid, Trp-Met-Asp-Phe-NH2; ili PTK7) je peptidni fragment izveden iz većeg peptidnog hormona holecistokinina. Za razliku od holecistokina koji ima niz uloga u gastrointestinalnom sistemu kao i u centralnom nervnom sistemu, CCK-4 deluje prvenstveno u mozgu kao stimulator anksioznosti. On pokazuje slabe GI efekte, za razliku od CCK-8 ili polipeptida pune dužine, CCK-58.
CCK-4 proizvodi jake simptome anksioznosti u malim dozama, kao što je 50 μg,[1] i često se koristi u naučnim istraživanjima za indukovanje paničnih napada s ciljem testiranja novih anksiolitika.[2][3][4][5] Pošto je on peptid, CCK-4 mora biti administriran putem injekcije. U telu se brzo razlaže, tako da ima kratkotrajno dejstvo.[6] Brojni sintetički analozi sa modifikovanim osobinama su poznati.[7][8][9][10][11][12][13][14][15][16][17]
Vidi još[uredi | uredi izvor]
Reference[uredi | uredi izvor]
- ^ Daniela Eser; et al. (2005). „Panic Induction with Cholecystokinin-Tetrapeptide (CCK-4) Increases Plasma Concentrations of the Neuroactive Steroid 3α, 5α Tetrahydrodeoxycorticosterone (3α, 5α-THDOC) in Healthy Volunteers” (PDF). Neuropsychopharmacology. 30 (1): 192—195. PMID 15467707. doi:10.1038/sj.npp.1300572.
- ^ Bradwejn J. (1993). „Neurobiological investigations into the role of cholecystokinin in panic disorder”. Journal of Psychiatry and Neuroscience. 18 (4): 178—188. PMC 1188527
 . PMID 8104032.
. PMID 8104032.
- ^ Schunck T, Erb G, Mathis A, Gilles C, Namer IJ, Hode Y, Demaziere A, Luthringer R, Macher JP (2006). „Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety”. NeuroImage. 31 (3): 1197—1208. PMID 16600640. doi:10.1016/j.neuroimage.2006.01.035.
- ^ Eser D, Schüle C, Baghai T, Floesser A, Krebs-Brown A, Enunwa M, de la Motte S, Engel R, Kucher K, Rupprecht R (2007). „Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study”. Psychopharmacology. 192 (4): 479—487. PMID 17318504. doi:10.1007/s00213-007-0738-7.
- ^ Eser D, Leicht G, Lutz J, Wenninger S, Kirsch V, Schüle C, Karch S, Baghai T, Pogarell O, Born C, Rupprecht R, Mulert C (2007). „Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers”. Human Brain Mapping. 30 (2): 511—22. PMID 18095276. doi:10.1002/hbm.20522.
- ^ Koulischer D, Moroder L, Deschodt-Lanckman M (1982). „Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro”. Regulatory Peptides. 4 (3): 127—139. PMID 6291099. doi:10.1016/0167-0115(82)90080-5.
- ^ Blommaert AG, Dhôtel H, Ducos B, Durieux C, Goudreau N, Bado A, Garbay C, Roques BP (1997). „Structure-based design of new constrained cyclic agonists of the cholecystokinin CCK-B receptor”. Journal of Medicinal Chemistry. 40 (5): 647—58. PMID 9057851. doi:10.1021/jm9603072.
- ^ Bellier B, Million ME, DaNascimento S, Meudal H, Kellou S, Maigret B, Garbay C (2000). „Replacement of glycine with dicarbonyl and related moieties in analogues of the C-terminal pentapeptide of cholecystokinin: CCK(2) agonists displaying a novel binding mode”. Journal of Medicinal Chemistry. 43 (20): 3614—23. PMID 11020275. doi:10.1021/jm0000416.
- ^ Léna I, Dh tel H, Garbay C, Daugé V (2001). „Involvement of D2 dopamine receptors in the opposing effects of two CCK-B agonists in a spatial recognition memory task: role of the anterior nucleus accumbens”. Psychopharmacology. 153 (2): 170—9. PMID 11205416. doi:10.1007/s002130000517.
- ^ Bellier B, Garbay C (2003). „How a single inversion of configuration leads to a reversal of the binding mode: proposal of a novel arrangement of CCK2 ligands in their receptor, and contribution to the development of peptidomimetic or non-peptide CCK2 ligands”. European Journal of Medicinal Chemistry. 38 (7-8): 671—86. PMID 12932898. doi:10.1016/S0223-5234(03)00112-0.
- ^ Bellier B, Crété D, Million ME, Beslot F, Bado A, Garbay C, Daugé V (2004). „New CCK2 agonists confirming the heterogeneity of CCK2 receptors: characterisation of BBL454”. Naunyn-Schmiedeberg's Archives of Pharmacology. 370 (5): 404—13. PMID 15480577. doi:10.1007/s00210-004-0969-7.
- ^ Proskuriakova TV; Bespalova ZhD; Pal'keeva ME; Petrichenko OB; Pankratova NV; Shokhonova VA; Anokhina IP (2005). „[Biological activity of cholecystokinin-(30-33) tetrapeptide analogs]”. Bioorganicheskaia Khimiia (на језику: руски). 31 (2): 130—9. PMID 15889786.
- ^ Anokhina IP; Proskuriakova TV; Bespalova ZhD; Pal'keeva ME; Shokhonova VA; Petrichenko OB (2006). „[Effect of a cholecystokinin tetrapeptide analogue on opioid reception under acute and chronic morphine administration]”. Bioorganicheskaia Khimiia (на језику: руски). 32 (3): 276—83. PMID 16808170.
- ^ Agnes RS, Lee YS, Davis P, Ma SW, Badghisi H, Porreca F, Lai J, Hruby VJ (2006). „Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors”. Journal of Medicinal Chemistry. 49 (10): 2868—75. PMC 1484468
 . PMID 16686530. doi:10.1021/jm050921q.
. PMID 16686530. doi:10.1021/jm050921q.
- ^ Noble F (2007). „Pharmacology of CCKRs and SAR studies of peptidic analog ligands”. Current Topics in Medicinal Chemistry. 7 (12): 1173—9. PMID 17584139. doi:10.2174/156802607780960447.
- ^ García-López MT, González-Muñiz R, Martín-Martínez M, Herranz R (2007). „Strategies for design of non peptide CCK1R agonist/antagonist ligands”. Current Topics in Medicinal Chemistry. 7 (12): 1180—94. PMID 17584140. doi:10.2174/156802607780960537.
- ^ Kalindjian SB, McDonald IM (2007). „Strategies for the design of non-peptide CCK2 receptor agonist and antagonist ligand”. Current Topics in Medicinal Chemistry. 7 (12): 1195—204. PMID 17584141. doi:10.2174/156802607780960500.
Spoljašnje veze[uredi | uredi izvor]
 | Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |
