S Vikipedije, slobodne enciklopedije
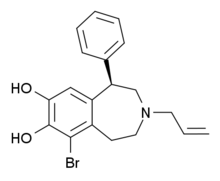
6-Br-APB 3-alil-6-bromo-1-fenil-1,2,4,5-tetrahidro-3-benzazepin-7,8-diol
CAS broj 135974-57-1 Н ATC kod none PubChem CID 11957483 ChemSpider 8627436 Y ChEMBL CHEMBL34095 Y Formula C 19 H 20 Br N O 2 Molarna masa 374,27 g/mol
c3ccccc3C2CN(CC=C)CCc(c1Br)c2cc(O)c1O
InChI=1S/C19H20BrNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1
Y Key:KKZGFVAZUKHFAC-MRXNPFEDSA-N
Y
6-Br-APB je sintetičko jedinjenje koje deluje kao selektivni agonist D1 receptora.[1] enantiomer je potentan pun agonist , dok (S) enantiomer ima D1 selektivnost, ali je slab parcijalni agonist .[2] 1 -selektivni puni agonisti, kao što su SKF-81297 i SKF-82958 proizvode karakteristične anoreksične efekte, stereotipno ponašanje i samoadministraciju kod životinja. On ima sličan mada ne identičan profile sa dopaminergičkim stimulansima poput amfetamina .[3] [4] [5]
^ Neumeyer JL, Baindur N, Niznik HB, Guan HC, Seeman P (1991). „(+/-)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3- benzazepin, a new high-affinity D1 dopamine receptor ligand: synthesis and structure-activity relationship”. Journal of Medicinal Chemistry . 34 (12): 3366—71. PMID 1684995 . doi :10.1021/jm00116a004 . ^ Neumeyer JL, Kula NS, Baldessarini RJ, Baindur N (1992). „Stereoisomeric probes for the D1 dopamine receptor: synthesis and characterization of R-(+) and S-(-) enantiomers of 3-allyl-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine and its 6-bromo analogue”. Journal of Medicinal Chemistry . 35 (8): 1466—71. PMID 1533424 . doi :10.1021/jm00086a016 . ^ Rosenzweig-Lipson S, Hesterberg P, Bergman J (1994). „Observational studies of dopamine D1 and D2 agonists in squirrel monkeys” . Psychopharmacology . 116 (1): 9—18. PMID 7862937 . doi :10.1007/BF02244865 . ^ Weed MR, Woolverton WL (1995). „The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys”. The Journal of Pharmacology and Experimental Therapeutics . 275 (3): 1367—74. PMID 8531104 . ^ Barrett AC, Miller JR, Dohrmann JM, Caine SB (2004). „Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats”. Neuropharmacology . 47 Suppl 1: 256—73. PMID 15464142 . doi :10.1016/j.neuropharm.2004.07.007 .
Morfolini :
Fenbutrazat •
Morazon •
Fendimetrazin •
Fenmetrazin ;
Oksazolini :
4-Metilaminoreks (4-MAR, 4-MAX) •
Aminoreks •
Klominoreks •
Ciklazodon •
Fenozolon •
Fluminoreks •
Pemolin •
Tozalinon ;
Fenetilamini (takođe
amfetamini ,
katinoni ,
fentermini , itd):
2-Hidroksifenetilamin (2-OH-PEA) •
4-CAB •
4-Metilamfetamin (4-MA) •
4-Metilmetamfetamin (4-MMA) •
Alfetamin •
Amfekloral •
Amfepentoreks •
Amfepramon •
Amfetamin (
Dekstroamfetamin •
Levoamfetamin ) •
Amfetaminil •
β-Metilfenetilamin (β-Me-PEA) •
Benzodioksolilbutanamin (BDB) •
Benzodioksolilhidroksibutanamin (BOH) •
Benzfetamin •
Bufedron •
Butilon •
Katin •
Katinon •
Klobenzoreks •
Klortermin •
D-deprenil •
Dimetoksiamfetamin (DMA) •
Dimetoksimetamfetamin (DMMA) •
Dimetilamfetamin •
Dimetilkatinon (Dimetilpropion, metamfepramon) •
Etkatinon (Etilpropion) •
Etilamfetamin •
Etilbenzodioksolilbutanamin (EBDB) •
Etilon •
Famprofazon •
Fenetilin •
Fenproporeks •
Flefedron •
Fludoreks •
Furfenoreks •
Hordenin •
Lofofin (Homomiristicilamin) •
Mefenoreks •
Mefedron •
Metamfetamin (Dezoksiefedrin, Metedrin;
Dekstrometamfetamin •
Levometamfetamin ) •
Metkatinon (Metilpropion) •
Metedron •
Metoksimetilendioksiamfetamin (MMDA) •
Metoksimetilenedioksimetamfetamin (MMDMA) •
Metilbenzodioksolilbutanamin (MBDB) •
Metilendioksiamfetamin (MDA, tenamfetamin) •
Metilendioksietilamfetamin (MDEA) •
Metilendioksihidroksiamfetamin (MDOH) •
Metilendioksimetamfetamin (MDMA) •
Metilendioksimetilfenetilamin (MDMPEA, homarilamin) •
Metilendioksifenetilamin (MDPEA, homopiperonilamin) •
Metilon •
Ortetamin •
Para-bromoamfetamin (PBA) •
Para-hloroamfetamin (PCA) •
Parafluoroamfetamin (PFA) •
Parafluorometamfetamin (PFMA) •
Parahidroksiamfetamin (PHA) •
Parajodoamfetamin (PIA) •
Paredrin (Norfoledrin, Oksamfetamin) •
Fenetilamin (PEA) •
Foledrin •
Fenprometamin •
Prenilamin •
Propilamfetamin •
Tifloreks (Flutioreks) •
Tiramin (TRA) •
Ksilopropamin •
Zilofuramin ;
Piperazini :
2,5-Dimetoksi-4-bromobenzilpiperazin (2C-B-BZP) •
Benzilpiperazin (BZP) •
Metoksifenilpiperazin (MeOPP, paraperazin) •
Metilbenzilpiperazin (MBZP) •
Metilendioksibenzilpiperazin (MDBZP, piperonilpiperazin);
Drugi :
2-Amino-1,2-dihidronaftalen (2-ADN) •
2-Aminoindan (2-AI) •
2-Aminotetralin (2-AT) •
4-Benzilpiperidin (4-BP) •
5-IAI •
Klofenciklan •
Ciklopentamin •
Cipenamin •
Ciprodenat •
Feprosidnin •
Gilutensin •
Heptaminol •
Heksaciklonat •
Indanilaminopropan (IAP) •
Indanoreks •
Izometepten •
Metilheksanamin •
Naftilaminopropan (NAP) •
Oktodrin •
Ftalimidopropiofenon •
Propilheksedrin (
Levopropilheksedrin ) •
Tuaminoheptan (Tuamin)
Molimo Vas, obratite pažnju na važno upozorenje oblasti medicine (zdravlja) .