Растварање — разлика између измена
. |
|||
| Ред 1: | Ред 1: | ||
[[Датотека:SaltInWaterSolutionLiquid.jpg|мини|десно|200п|Раствор кухињске соли у води]] |
[[Датотека:SaltInWaterSolutionLiquid.jpg|мини|десно|200п|Раствор кухињске соли у води]] |
||
'''Растварање''' је процес диспереговања (равномерног разређивања, распоређивања) једне [[хемијска супстанца|супстанце]] или више њих у другој. Осим код правих [[смеше|смеша]], увек је праћено разменом енергије са околином.<ref name="Atkins7th">{{Atkins7th}}</ref><ref name="McQuarrie1st">{{McQuarrie1st}}</ref> |
'''Растварање''' је процес диспереговања (равномерног разређивања, распоређивања) једне [[хемијска супстанца|супстанце]] или више њих у другој. Осим код правих [[смеше|смеша]], увек је праћено разменом енергије са околином.<ref name="Atkins7th">{{Atkins7th}}</ref><ref name="McQuarrie1st">{{McQuarrie1st}}</ref> |
||
{{рут}} |
|||
Растварач је супстанца која раствара растворак, чиме се формира [[раствор]]. A solvent is usually a liquid but can also be a solid, a gas, or a [[supercritical fluid]]. The quantity of solute that can dissolve in a specific volume of solvent varies with [[temperature]]. Common uses for [[Organic compound|organic]] solvents are in [[dry cleaning]] (e.g. [[tetrachloroethylene]]), as [[paint thinner]]s (e.g. [[toluene]], [[turpentine]]), as nail polish removers and glue solvents ([[acetone]], [[methyl acetate]], [[ethyl acetate]]), in spot removers (e.g. [[hexane]], petrol ether), in detergents ([[D-limonene|citrus terpenes]]) and in [[perfume]]s ([[ethanol]]). Water is a solvent for [[Chemical polarity#Polarity of molecules|polar molecules]] and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents find various applications in chemical, [[pharmaceutical]], oil, and gas industries, including in [[Chemical synthesis|chemical syntheses]] and purification processes. |
|||
== Растварач == |
== Растварач == |
||
| Ред 13: | Ред 15: | ||
== Растворљивост == |
== Растворљивост == |
||
'''Растворљивост''' неке супстанце представља број грама супстанције који се раствара у 100г растварача на одређеној темпаратури. |
'''Растворљивост''' неке супстанце представља број грама супстанције који се раствара у 100г растварача на одређеној темпаратури. |
||
== Вишекомпонентни == |
|||
=== Растварачи === |
|||
{| class="wikitable" |
|||
|- |
|||
! Име !! Композиција |
|||
|- |
|||
| Solvent 645 || toluene 50%, butyl acetate 18%, ethyl acetate 12%, butanol 10%, ethanol 10%. |
|||
|- |
|||
| Solvent 646 || [[toluene]] 50%, [[ethanol]] 15%, [[butanol]] 10%, butyl- or [[amyl acetate]] 10%, [[ethyl cellosolve]] 8%, [[acetone]] 7%<ref>[https://www.dcpt.ru/rastvoritel-646/#tab3 dcpt.ru Solvent 646 Characteristics (ru)]</ref> |
|||
|- |
|||
| Solvent 647 || butyl- or amyl acetate 29.8%, [[ethyl acetate]] 21.2%, butanol 7.7%, toluene or [[pyrobenzene]] 41.3%<ref>[https://www.dcpt.ru/rastvoritel-647/#tab3 dcpt.ru Solvent 647 Characteristics (ru)]</ref> |
|||
|- |
|||
| Solvent 648 || [[butyl acetate]] 50%, ethanol 10%, butanol 20%, toluene 20%<ref>[https://www.dcpt.ru/rastvoritel-marki-r-648/ dcpt.ru Solvent 648 Characteristics (ru)]</ref> |
|||
|- |
|||
| Solvent 649 || ethyl cellosolve 30%, butanol 20%, [[xylene]] 50% |
|||
|- |
|||
| Solvent 650 || ethyl cellosolve 20%, butanol 30%, xylene 50%<ref>[https://www.dcpt.ru/primenenie-r-650-ximicheskogo-rastvoritelya/ dcpt.ru Solvent 650 Characteristics (ru)]</ref> |
|||
|- |
|||
| Solvent 651 || [[white spirit]] 90%, butanol 10% |
|||
|- |
|||
| Solvent KR-36 || butyl acetate 20%, butanol 80% |
|||
|- |
|||
| Solvent P-4 || toluene 62%, acetone 26%, butyl acetate 12%. |
|||
|- |
|||
| Solvent P-10 || xylene 85%, acetone 15%. |
|||
|- |
|||
| Solvent P-12 || toluene 60%, butyl acetate 30%, xylene 10%. |
|||
|- |
|||
| Solvent P-14 || cyclohexanone 50%, toluene 50%. |
|||
|- |
|||
| Solvent P-24 || solvent 50%, xylene 35%, acetone 15%. |
|||
|- |
|||
| Solvent P-40 || toluene 50%, ethyl cellosolve 30%, acetone 20%. |
|||
|- |
|||
| Solvent P-219 || toluene 34%, cyclohexanone 33%, acetone 33%. |
|||
|- |
|||
| Solvent P-3160 || butanol 60%, ethanol 40%. |
|||
|- |
|||
| Solvent RCC || xylene 90%, butyl acetate 10%. |
|||
|- |
|||
| Solvent RML || ethanol 64%, ethylcellosolve 16%, toluene 10%, butanol 10%. |
|||
|- |
|||
| Solvent PML-315 || toluene 25%, xylene 25%, butyl acetate 18%, ethyl cellosolve 17%, butanol 15%. |
|||
|- |
|||
| Solvent PC-1 || toluene 60%, butyl acetate 30%, xylene 10%. |
|||
|- |
|||
| Solvent PC-2 || white spirit 70%, xylene 30%. |
|||
|- |
|||
| Solvent RFG || ethanol 75%, butanol 25%. |
|||
|- |
|||
| Solvent RE-1 || xylene 50%, acetone 20%, butanol 15%, ethanol 15%. |
|||
|- |
|||
| Solvent RE-2 || Solvent 70%, ethanol 20%, acetone 10%. |
|||
|- |
|||
| Solvent RE-3 || solvent 50%, ethanol 20%, acetone 20%, ethyl cellosolve 10%. |
|||
|- |
|||
| Solvent RE-4 || solvent 50%, acetone 30%, ethanol 20%. |
|||
|- |
|||
| Solvent FK-1 (?) || absolute alcohol (99.8%) 95%, ethyl acetate 5% |
|||
|} |
|||
=== Разређивачи === |
|||
{| class="wikitable" |
|||
|- |
|||
! Име !! Композиција |
|||
|- |
|||
|Thinner RKB-1 || butanol 50%, xylene 50% |
|||
|- |
|||
| Thinner RKB-2 || butanol 95%, xylene 5% |
|||
|- |
|||
| Thinner RKB-3 || xylene 90%, butanol 10% |
|||
|- |
|||
| Thinner M || ethanol 65%, butyl acetate 30%, ethyl acetate 5%. |
|||
|- |
|||
| Thinner P-7 || cyclohexanone 50%, ethanol 50%. |
|||
|- |
|||
| Thinner R-197 || xylene 60%, butyl acetate 20%, ethyl cellosolve 20%. |
|||
|- |
|||
| Thinner of WFD || toluene 50%, butyl acetate (or amyl acetate) 18%, butanol 10%, ethanol 10%, ethyl acetate 9%, acetone 3%. |
|||
|} |
|||
== Физичка својства == |
|||
=== Табела својстава уобичајених растварача === |
|||
The solvents are grouped into [[nonpolar]], polar [[aprotic]], and polar [[protic]] solvents, with each group ordered by increasing polarity. The [[Property|properties]] of solvents which exceed those of water are bolded. |
|||
<!-- Here is a table of data; skip past it to edit the text. --> |
|||
{| border="1" cellpadding="5" cellspacing="0" style="margin:auto; text-align:center;" class="wikitable" |
|||
|- |
|||
! Solvent |
|||
! [[Chemical formula]] |
|||
! [[Boiling point]]<ref name=boil>[http://www.xydatasource.com/xy-showdatasetpage.php?datasetcode=9724855&dsid=1138&searchtext=solvent Solvent Properties – Boiling Point] {{webarchive|url=https://web.archive.org/web/20110614130546/http://www.xydatasource.com/xy-showdatasetpage.php?datasetcode=9724855&dsid=1138&searchtext=solvent |date=14 June 2011 }}. Xydatasource.com. Retrieved on 26 January 2013.</ref><br/>(°C) |
|||
! [[Dielectric constant]]<ref>[http://macro.lsu.edu/HowTo/solvents/Dielectric%20Constant%20.htm Dielectric Constant] {{webarchive|url=https://web.archive.org/web/20100704013154/http://macro.lsu.edu/HowTo/solvents/Dielectric%20Constant%20.htm |date=4 July 2010 }}. Macro.lsu.edu. Retrieved on 26 January 2013.</ref> |
|||
! [[Density]]<br/>(g/mL) |
|||
! [[Molecular dipole moment|Dipole moment]]<br/>([[Debye|D]]) |
|||
<!-- ### Non-polar solvents ### --> |
|||
|- style="background:#ddd;height: 50px;vertical-align:top" |
|||
! colspan="6"| |
|||
==== [[Nonpolar]] solvents ==== |
|||
|- style="background:#ddd;" |
|||
| [[Pentane]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> |
|||
| 36 |
|||
| 1.84 |
|||
| 0.626 |
|||
| 0.00 |
|||
|- style="background:#ddd;" |
|||
| [[Cyclopentane]] |
|||
| [[File:Cyclopentane 200.svg|100px]]<br/>C<sub>5</sub>H<sub>10</sub> |
|||
| 40 |
|||
| 1.97 |
|||
| 0.751 |
|||
| 0.00 |
|||
|- style="background:#ddd;" |
|||
| [[Hexane]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> |
|||
| 69 |
|||
| 1.88 |
|||
| 0.655 |
|||
| 0.00 |
|||
|- style="background:#ddd;" |
|||
| [[Cyclohexane]] |
|||
| [[File:Cyclohexane-2D-skeletal.svg|90px]]<br/>C<sub>6</sub>H<sub>12</sub> |
|||
| 81 |
|||
| 2.02 |
|||
| 0.779 |
|||
| 0.00 |
|||
|- style="background:#ddd;" |
|||
| [[Benzene]] |
|||
| [[File:Benzene 200.svg|90px]]<br/>C<sub>6</sub>H<sub>6</sub> |
|||
| 80 |
|||
| 2.3 |
|||
| 0.879 |
|||
| 0.00 |
|||
|- style="background:#ddd;" |
|||
| [[Toluene]] |
|||
| C<sub>6</sub>H<sub>5</sub>-CH<sub>3</sub> |
|||
| '''111''' |
|||
| 2.38 |
|||
| 0.867 |
|||
| 0.36 |
|||
|- style="background:#ddd;" |
|||
| [[1,4-Dioxane]] |
|||
| [[File:1-4-Dioxane.svg|100px]]<br/>C<sub>4</sub>H<sub>8</sub>O<sub>2</sub> |
|||
| '''101''' |
|||
| 2.3 |
|||
| '''1.033''' |
|||
| 0.45 |
|||
|- style="background:#ddd;" |
|||
| [[Chloroform]] |
|||
| CHCl<sub>3</sub> |
|||
| 61 |
|||
| 4.81 |
|||
| '''1.498''' |
|||
| 1.04 |
|||
|- style="background:#ddd;" |
|||
| [[Diethyl ether]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>-O-CH<sub>2</sub>CH<sub>3</sub> |
|||
| 35 |
|||
| 4.3 |
|||
| 0.713 |
|||
| 1.15 |
|||
|- style="background:#ddd;" |
|||
| [[Dichloromethane]] (DCM) |
|||
| CH<sub>2</sub>Cl<sub>2</sub> |
|||
| 40 |
|||
| 9.1 |
|||
| '''1.3266''' |
|||
| 1.60 |
|||
|- style="background:#ddd;" |
|||
<!-- ### Polar aprotic solvents ### --> |
|||
|- style="background:#fcf;height: 50px;vertical-align:top" |
|||
! colspan="6"| |
|||
==== [[Chemical polarity|Поларни]] [[Polar solvent|апротични]] растварачи ==== |
|||
|- style="background:#fcf;" |
|||
| [[Tetrahydrofuran]] (THF) |
|||
| [[File:Tetrahydrofuran.svg|100px]]<br/>C<sub>4</sub>H<sub>8</sub>O |
|||
| 66 |
|||
| 7.5 |
|||
| 0.886 |
|||
| 1.75 |
|||
|- style="background:#fcf;" |
|||
| [[Ethyl acetate]] |
|||
| [[File:Essigsäureethylester.svg]]<br/>CH<sub>3</sub>-C(=O)-O-CH<sub>2</sub>-CH<sub>3</sub> |
|||
| 77 |
|||
| 6.02 |
|||
| 0.894 |
|||
| 1.78 |
|||
|- style="background:#fcf;" |
|||
| [[Acetone]] |
|||
| [[File:Acetone-2D-skeletal.svg|90px]]<br/>CH<sub>3</sub>-C(=O)-CH<sub>3</sub> |
|||
| 56 |
|||
| 21 |
|||
| 0.786 |
|||
| '''2.88''' |
|||
|- style="background:#fcf;" |
|||
| [[Dimethylformamide]] (DMF) |
|||
| [[File:Dimethylformamide.svg|100px]]<br/>H-C(=O)N(CH<sub>3</sub>)<sub>2</sub> |
|||
| '''153''' |
|||
| 38 |
|||
| 0.944 |
|||
| '''3.82''' |
|||
|- style="background:#fcf;" |
|||
| [[Acetonitrile]] (MeCN) |
|||
| CH<sub>3</sub>-C≡N |
|||
| 82 |
|||
| 37.5 |
|||
| 0.786 |
|||
| '''3.92''' |
|||
|- style="background:#fcf;" |
|||
| [[Dimethyl sulfoxide]] (DMSO) |
|||
| [[File:Dimethylsulfoxid.svg]]<br/>CH<sub>3</sub>-S(=O)-CH<sub>3</sub> |
|||
| '''189''' |
|||
| 46.7 |
|||
| '''1.092''' |
|||
| '''3.96''' |
|||
|- style="background:#fcf;" |
|||
| [[Nitromethane]] |
|||
| CH<sub>3</sub>-NO<sub>2</sub> |
|||
| '''100–103''' |
|||
| 35.87 |
|||
| '''1.1371''' |
|||
| '''3.56''' |
|||
|- style="background:#fcf;" |
|||
| [[Propylene carbonate]] |
|||
| C<sub>4</sub>H<sub>6</sub>O<sub>3</sub> |
|||
| '''240''' |
|||
| 64.0 |
|||
| '''1.205''' |
|||
| '''4.9''' |
|||
<!-- ### Polar protic solvents ### --> |
|||
|- style="background:#fcc;height: 50px;vertical-align:top" |
|||
! colspan="6"| |
|||
==== Поларни [[Polar solvent|протични]] растварачи ==== |
|||
|- style="background:#fcc;" |
|||
| [[Formic acid]] |
|||
| [[File:Formic acid.svg|100px]]<br/>H-C(=O)OH |
|||
| '''101''' |
|||
| 58 |
|||
| '''1.21''' |
|||
| 1.41 |
|||
|- style="background:#fcc;" |
|||
| [[n-Butanol|''n''-Butanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH |
|||
| '''118''' |
|||
| 18 |
|||
| 0.810 |
|||
| 1.63 |
|||
|- style="background:#fcc;" |
|||
| [[Isopropyl alcohol]] (IPA) |
|||
| [[File:2-Propanol2.svg|100px]]<br/>CH<sub>3</sub>-CH(-OH)-CH<sub>3</sub> |
|||
| 82 |
|||
| 18 |
|||
| 0.785 |
|||
| 1.66 |
|||
|- style="background:#fcc;" |
|||
| [[1-Propanol|''n''-Propanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH |
|||
| 97 |
|||
| 20 |
|||
| 0.803 |
|||
| 1.68 |
|||
|- style="background:#fcc;" |
|||
| [[Ethanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>OH |
|||
| 79 |
|||
| 24.55 |
|||
| 0.789 |
|||
| 1.69 |
|||
|- style="background:#fcc;" |
|||
| [[Methanol]] |
|||
| CH<sub>3</sub>OH |
|||
| 65 |
|||
| 33 |
|||
| 0.791 |
|||
| 1.70 |
|||
|- style="background:#fcc;" |
|||
| [[Acetic acid]] |
|||
| [[File:Essigsäure - Acetic acid.svg|100px]]<br/>CH<sub>3</sub>-C(=O)OH |
|||
| '''118''' |
|||
| 6.2 |
|||
| '''1.049''' |
|||
| 1.74 |
|||
|- style="background:#fcc;" |
|||
| [[Water (molecule)|Water]] |
|||
| [[File:Wasser Strukturformel V1.svg|75px]]<br/>H-O-H |
|||
| 100 |
|||
| 80 |
|||
| 1.000 |
|||
| 1.85 |
|||
|} |
|||
=== Вредности параметра Хансенове растворљивости === |
|||
The Hansen solubility parameter values<ref name=hansen/><ref name=hansen2/> are based on [[Van der Waals forces|dispersion bonds]] (δD), [[polar bonds]] (δP) and [[hydrogen bonds]] (δH). These contain information about the inter-molecular interactions with other solvents and also with polymers, pigments, nanoparticles, etc. This allows for rational formulations knowing, for example, that there is a good HSP match between a solvent and a polymer. Rational substitutions can also be made for "good" solvents (effective at dissolving the solute) that are "bad" (expensive or hazardous to health or the environment). The following table shows that the intuitions from "non-polar", "polar aprotic" and "polar protic" are put numerically – the "polar" molecules have higher levels of δP and the protic solvents have higher levels of δH. Because numerical values are used, comparisons can be made rationally by comparing numbers. For example, acetonitrile is much more polar than acetone but exhibits slightly less hydrogen bonding. |
|||
<!-- Here is a table of data; skip past it to edit the text. --> |
|||
{| border="1" cellpadding="5" cellspacing="0" style="margin:auto; text-align:center;" class="wikitable" |
|||
|- |
|||
! Растварач |
|||
! [[Chemical formula]] |
|||
! δD Dispersion |
|||
! δP Polar |
|||
! δH Hydrogen bonding |
|||
<!-- ### Non-polar solvents ### --> |
|||
|- style="background:#ddd;height: 50px;vertical-align:top" |
|||
! colspan="5"| |
|||
==== Неполарни растварачи ==== |
|||
|- style="background:#ddd;" |
|||
| [[n-Hexane]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> |
|||
| 14.9 |
|||
| 0.0 |
|||
| 0.0 |
|||
|- style="background:#ddd;" |
|||
| [[Benzene]] |
|||
| C<sub>6</sub>H<sub>6</sub> |
|||
| 18.4 |
|||
| 0.0 |
|||
| 2.0 |
|||
|- style="background:#ddd;" |
|||
| [[Toluene]] |
|||
| C<sub>6</sub>H<sub>5</sub>-CH<sub>3</sub> |
|||
| 18.0 |
|||
| 1.4 |
|||
| 2.0 |
|||
|- style="background:#ddd;" |
|||
| [[Diethyl ether]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>-O-CH<sub>2</sub>CH<sub>3</sub> |
|||
| 14.5 |
|||
| 2.9 |
|||
| 4.6 |
|||
|- style="background:#ddd;" |
|||
| [[Chloroform]] |
|||
| CHCl<sub>3</sub> |
|||
| 17.8 |
|||
| 3.1 |
|||
| 5.7 |
|||
|- style="background:#ddd;" |
|||
| [[1,4-Dioxane]] |
|||
| <u>/-CH<sub>2</sub>-CH<sub>2</sub>-O-CH<sub>2</sub>-CH<sub>2</sub>-O-\</u> |
|||
| 17.5 |
|||
| 1.8 |
|||
| 9.0 |
|||
|- style="background:#fcf;height: 50px;vertical-align:top"<!-- ### Polar aprotic solvents ### --> |
|||
! colspan="5"| |
|||
==== Поларни апротични растварачи ==== |
|||
|- style="background:#fcf;" |
|||
| [[Ethyl acetate]] |
|||
| CH<sub>3</sub>-C(=O)-O-CH<sub>2</sub>-CH<sub>3</sub> |
|||
| 15.8 |
|||
| 5.3 |
|||
| 7.2 |
|||
|- style="background:#fcf;" |
|||
| [[Tetrahydrofuran]] (THF) |
|||
| <u>/-CH<sub>2</sub>-CH<sub>2</sub>-O-CH<sub>2</sub>-CH<sub>2</sub>-\</u> |
|||
| 16.8 |
|||
| 5.7 |
|||
| 8.0 |
|||
|- style="background:#fcf;" |
|||
| [[Dichloromethane]] |
|||
| CH<sub>2</sub>Cl<sub>2</sub> |
|||
| 17.0 |
|||
| 7.3 |
|||
| 7.1 |
|||
|- style="background:#fcf;" |
|||
| [[Acetone]] |
|||
| CH<sub>3</sub>-C(=O)-CH<sub>3</sub> |
|||
| 15.5 |
|||
| 10.4 |
|||
| 7.0 |
|||
|- style="background:#fcf;" |
|||
| [[Acetonitrile]] (MeCN) |
|||
| CH<sub>3</sub>-C≡N |
|||
| 15.3 |
|||
| 18.0 |
|||
| 6.1 |
|||
|- style="background:#fcf;" |
|||
| [[Dimethylformamide]] (DMF) |
|||
| H-C(=O)N(CH<sub>3</sub>)<sub>2</sub> |
|||
| 17.4 |
|||
| 13.7 |
|||
| 11.3 |
|||
|- style="background:#fcf;" |
|||
| [[Dimethyl sulfoxide]] (DMSO) |
|||
| CH<sub>3</sub>-S(=O)-CH<sub>3</sub> |
|||
| 18.4 |
|||
| 16.4 |
|||
| 10.2 |
|||
<!-- ### Polar protic solvents ### --> |
|||
|- style="background:#fcc;height: 50px;vertical-align:top" |
|||
! colspan="5"| |
|||
==== Поларни протични растварачи ==== |
|||
|- style="background:#fcc;" |
|||
| [[Acetic acid]] |
|||
| CH<sub>3</sub>-C(=O)OH |
|||
| 14.5 |
|||
| 8.0 |
|||
| 13.5 |
|||
|- style="background:#fcc;" |
|||
| [[n-Butanol|''n''-Butanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH |
|||
| 16.0 |
|||
| 5.7 |
|||
| 15.8 |
|||
|- style="background:#fcc;" |
|||
| [[Isopropanol]] |
|||
| CH<sub>3</sub>-CH(-OH)-CH<sub>3</sub> |
|||
| 15.8 |
|||
| 6.1 |
|||
| 16.4 |
|||
|- style="background:#fcc;" |
|||
| [[1-Propanol|''n''-Propanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH |
|||
| 16.0 |
|||
| 6.8 |
|||
| 17.4 |
|||
|- style="background:#fcc;" |
|||
| [[Ethanol]] |
|||
| CH<sub>3</sub>CH<sub>2</sub>OH |
|||
| 15.8 |
|||
| 8.8 |
|||
| 19.4 |
|||
|- style="background:#fcc;" |
|||
| [[Methanol]] |
|||
| CH<sub>3</sub>OH |
|||
| 14.7 |
|||
| 12.3 |
|||
| 22.3 |
|||
|- style="background:#fcc;" |
|||
| [[Formic acid]] |
|||
| H-C(=O)OH |
|||
| 14.6 |
|||
| 10.0 |
|||
| 14.0 |
|||
|- style="background:#fcc;" |
|||
| [[Water (molecule)|Water]] |
|||
| H-O-H |
|||
| 15.5 |
|||
| 16.0 |
|||
| 42.3 |
|||
|- |
|||
|} |
|||
If, for environmental or other reasons, a solvent or solvent blend is required to replace another of equivalent solvency, the substitution can be made on the basis of the [[Hansen solubility parameters]] of each. The values for mixtures are taken as the [[weighted average]]s of the values for the neat solvents. This can be calculated by [[trial-and-error]], a spreadsheet of values, or HSP software.<ref name=hansen>{{cite book | vauthors = Abbott S, Hansen CM | title = Hansen solubility parameters in practice. | publisher = Hansen-Solubility | date = 2008 | isbn = 978-0-9551220-2-6 | url = https://books.google.com/books?id=efMbTvlfc8wC&printsec=frontcover#v=onepage&q=&f=false }}</ref><ref name=hansen2>{{cite book | vauthors = Hansen CM | title = Hansen solubility parameters: a user's handbook. | publisher = CRC press | date = January 2002 | isbn = 978-0-8493-7248-3 | url = https://books.google.com/books?id=gprF31cvT2oC&printsec=frontcover }}</ref> A 1:1 mixture of [[toluene]] and [[1,4 dioxane]] has δD, δP and δH values of 17.8, 1.6 and 5.5, comparable to those of [[chloroform]] at 17.8, 3.1 and 5.7 respectively. Because of the health hazards associated with toluene itself, other mixtures of solvents may be found using a full [[Hansen solubility parameter|HSP]] dataset. |
|||
== Види још == |
== Види још == |
||
| Ред 20: | Ред 475: | ||
== Референце == |
== Референце == |
||
{{reflist| |
{{reflist|}} |
||
== Литература == |
== Литература == |
||
{{refbegin|30em}} |
|||
* {{cite book | vauthors = Lowery TH, Richardson KS | title = Mechanism and Theory in Organic Chemistry | publisher = [[Harper Collins Publishers]] | edition = 3rd | date = 1987 | isbn = 978-0-06-364044-3 }} |
|||
{{refend}} |
|||
== Спољашње везе == |
== Спољашње везе == |
||
{{Commonscat|Solvents}} |
{{Commonscat|Solvents}} |
||
* -{[http://www.esig.org "European Solvents Industry Group - ESIG - ESIG European Solvents Industry Group"] Solvents in Europe.}- |
|||
* -{[https://web.archive.org/web/20041113054037/http://www.usm.maine.edu/~newton/Chy251_253/Lectures/Solvents/Solvents.html Table and text] O-Chem Lecture}- |
|||
* -{[https://web.archive.org/web/20041207190740/http://virtual.yosemite.cc.ca.us/smurov/orgsoltab.htm Tables] Properties and toxicities of organic solvents}- |
|||
* -{[https://www.cdc.gov/niosh/topics/organsolv/ CDC – Organic Solvents – NIOSH Workplace Safety and Health Topic]}- |
|||
* -{[https://www.epa.gov/hwgenerators/final-rule-2013-conditional-exclusions-solid-waste-and-hazardous-waste-solvent EPA – Solvent Contaminated Wipes]}- |
|||
{{Реакциони механизми}} |
|||
{{Authority control}} |
|||
[[Категорија:Растварачи]] |
[[Категорија:Растварачи]] |
||
Верзија на датум 26. фебруар 2020. у 12:08

Растварање је процес диспереговања (равномерног разређивања, распоређивања) једне супстанце или више њих у другој. Осим код правих смеша, увек је праћено разменом енергије са околином.[1][2]
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
Растварач је супстанца која раствара растворак, чиме се формира раствор. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Common uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene), as paint thinners (e.g. toluene, turpentine), as nail polish removers and glue solvents (acetone, methyl acetate, ethyl acetate), in spot removers (e.g. hexane, petrol ether), in detergents (citrus terpenes) and in perfumes (ethanol). Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes.
Растварач
Растварач, релативан и конвенционални појам, назив за средину у којој је нека супстанца дисперегована. Идеалан раствор, смеша, без хемијског међудејства средине и растворене супстанце или са међудејством. Може бити чврст, течан или гасовит. Вода је универзалан растварач. Растварачи као вода веома су значајни за живот, такође и у индустрији, а нарочито важну улогу имају селективни растварачи.
Растварачи у дегазацији
Растварачи у дегазацији су органска једињења с двојаком наменом - за растварање дегазитора у току њихове припреме за употребу и за растварање самих бојних отрова, односно за дагазацију физичким путем. Као растварачи дегазатора најчешће се употребљавају дихлоретан, тетрахлоругљеник и титрохлоретилен, а као растварачи бојних отрова бензин и петролеум.
Раствор
Раствор (чврст, течан или гасовит) настаје мешањем двеју или више супстанци. Засићен раствор за дату температуру има максималну концетрацију растворене супстанце. Незасићен раствор је раствор у коме се може растворити још извесна количина већ растворене супстанце све док се не добије засићен простор. Презасићен простор је раствор у коме је растворено више супстанци него што одговара засићеном раствору. Нестабилан је и из њега се лако издваја, у облику талога, вишак растворене супстанце.
Растворљивост
Растворљивост неке супстанце представља број грама супстанције који се раствара у 100г растварача на одређеној темпаратури.
Вишекомпонентни
Растварачи
| Име | Композиција |
|---|---|
| Solvent 645 | toluene 50%, butyl acetate 18%, ethyl acetate 12%, butanol 10%, ethanol 10%. |
| Solvent 646 | toluene 50%, ethanol 15%, butanol 10%, butyl- or amyl acetate 10%, ethyl cellosolve 8%, acetone 7%[3] |
| Solvent 647 | butyl- or amyl acetate 29.8%, ethyl acetate 21.2%, butanol 7.7%, toluene or pyrobenzene 41.3%[4] |
| Solvent 648 | butyl acetate 50%, ethanol 10%, butanol 20%, toluene 20%[5] |
| Solvent 649 | ethyl cellosolve 30%, butanol 20%, xylene 50% |
| Solvent 650 | ethyl cellosolve 20%, butanol 30%, xylene 50%[6] |
| Solvent 651 | white spirit 90%, butanol 10% |
| Solvent KR-36 | butyl acetate 20%, butanol 80% |
| Solvent P-4 | toluene 62%, acetone 26%, butyl acetate 12%. |
| Solvent P-10 | xylene 85%, acetone 15%. |
| Solvent P-12 | toluene 60%, butyl acetate 30%, xylene 10%. |
| Solvent P-14 | cyclohexanone 50%, toluene 50%. |
| Solvent P-24 | solvent 50%, xylene 35%, acetone 15%. |
| Solvent P-40 | toluene 50%, ethyl cellosolve 30%, acetone 20%. |
| Solvent P-219 | toluene 34%, cyclohexanone 33%, acetone 33%. |
| Solvent P-3160 | butanol 60%, ethanol 40%. |
| Solvent RCC | xylene 90%, butyl acetate 10%. |
| Solvent RML | ethanol 64%, ethylcellosolve 16%, toluene 10%, butanol 10%. |
| Solvent PML-315 | toluene 25%, xylene 25%, butyl acetate 18%, ethyl cellosolve 17%, butanol 15%. |
| Solvent PC-1 | toluene 60%, butyl acetate 30%, xylene 10%. |
| Solvent PC-2 | white spirit 70%, xylene 30%. |
| Solvent RFG | ethanol 75%, butanol 25%. |
| Solvent RE-1 | xylene 50%, acetone 20%, butanol 15%, ethanol 15%. |
| Solvent RE-2 | Solvent 70%, ethanol 20%, acetone 10%. |
| Solvent RE-3 | solvent 50%, ethanol 20%, acetone 20%, ethyl cellosolve 10%. |
| Solvent RE-4 | solvent 50%, acetone 30%, ethanol 20%. |
| Solvent FK-1 (?) | absolute alcohol (99.8%) 95%, ethyl acetate 5% |
Разређивачи
| Име | Композиција |
|---|---|
| Thinner RKB-1 | butanol 50%, xylene 50% |
| Thinner RKB-2 | butanol 95%, xylene 5% |
| Thinner RKB-3 | xylene 90%, butanol 10% |
| Thinner M | ethanol 65%, butyl acetate 30%, ethyl acetate 5%. |
| Thinner P-7 | cyclohexanone 50%, ethanol 50%. |
| Thinner R-197 | xylene 60%, butyl acetate 20%, ethyl cellosolve 20%. |
| Thinner of WFD | toluene 50%, butyl acetate (or amyl acetate) 18%, butanol 10%, ethanol 10%, ethyl acetate 9%, acetone 3%. |
Физичка својства
Табела својстава уобичајених растварача
The solvents are grouped into nonpolar, polar aprotic, and polar protic solvents, with each group ordered by increasing polarity. The properties of solvents which exceed those of water are bolded.
| Solvent | Chemical formula | Boiling point[7] (°C) |
Dielectric constant[8] | Density (g/mL) |
Dipole moment (D) |
|---|---|---|---|---|---|
Nonpolar solvents | |||||
| Pentane | CH3CH2CH2CH2CH3 | 36 | 1.84 | 0.626 | 0.00 |
| Cyclopentane | 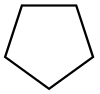 C5H10 |
40 | 1.97 | 0.751 | 0.00 |
| Hexane | CH3CH2CH2CH2CH2CH3 | 69 | 1.88 | 0.655 | 0.00 |
| Cyclohexane |  C6H12 |
81 | 2.02 | 0.779 | 0.00 |
| Benzene | 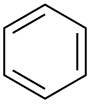 C6H6 |
80 | 2.3 | 0.879 | 0.00 |
| Toluene | C6H5-CH3 | 111 | 2.38 | 0.867 | 0.36 |
| 1,4-Dioxane |  C4H8O2 |
101 | 2.3 | 1.033 | 0.45 |
| Chloroform | CHCl3 | 61 | 4.81 | 1.498 | 1.04 |
| Diethyl ether | CH3CH2-O-CH2CH3 | 35 | 4.3 | 0.713 | 1.15 |
| Dichloromethane (DCM) | CH2Cl2 | 40 | 9.1 | 1.3266 | 1.60 |
Поларни апротични растварачи | |||||
| Tetrahydrofuran (THF) | 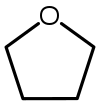 C4H8O |
66 | 7.5 | 0.886 | 1.75 |
| Ethyl acetate |  CH3-C(=O)-O-CH2-CH3 |
77 | 6.02 | 0.894 | 1.78 |
| Acetone |  CH3-C(=O)-CH3 |
56 | 21 | 0.786 | 2.88 |
| Dimethylformamide (DMF) | 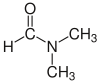 H-C(=O)N(CH3)2 |
153 | 38 | 0.944 | 3.82 |
| Acetonitrile (MeCN) | CH3-C≡N | 82 | 37.5 | 0.786 | 3.92 |
| Dimethyl sulfoxide (DMSO) |  CH3-S(=O)-CH3 |
189 | 46.7 | 1.092 | 3.96 |
| Nitromethane | CH3-NO2 | 100–103 | 35.87 | 1.1371 | 3.56 |
| Propylene carbonate | C4H6O3 | 240 | 64.0 | 1.205 | 4.9 |
Поларни протични растварачи | |||||
| Formic acid |  H-C(=O)OH |
101 | 58 | 1.21 | 1.41 |
| n-Butanol | CH3CH2CH2CH2OH | 118 | 18 | 0.810 | 1.63 |
| Isopropyl alcohol (IPA) |  CH3-CH(-OH)-CH3 |
82 | 18 | 0.785 | 1.66 |
| n-Propanol | CH3CH2CH2OH | 97 | 20 | 0.803 | 1.68 |
| Ethanol | CH3CH2OH | 79 | 24.55 | 0.789 | 1.69 |
| Methanol | CH3OH | 65 | 33 | 0.791 | 1.70 |
| Acetic acid |  CH3-C(=O)OH |
118 | 6.2 | 1.049 | 1.74 |
| Water | H-O-H |
100 | 80 | 1.000 | 1.85 |
Вредности параметра Хансенове растворљивости
The Hansen solubility parameter values[9][10] are based on dispersion bonds (δD), polar bonds (δP) and hydrogen bonds (δH). These contain information about the inter-molecular interactions with other solvents and also with polymers, pigments, nanoparticles, etc. This allows for rational formulations knowing, for example, that there is a good HSP match between a solvent and a polymer. Rational substitutions can also be made for "good" solvents (effective at dissolving the solute) that are "bad" (expensive or hazardous to health or the environment). The following table shows that the intuitions from "non-polar", "polar aprotic" and "polar protic" are put numerically – the "polar" molecules have higher levels of δP and the protic solvents have higher levels of δH. Because numerical values are used, comparisons can be made rationally by comparing numbers. For example, acetonitrile is much more polar than acetone but exhibits slightly less hydrogen bonding.
| Растварач | Chemical formula | δD Dispersion | δP Polar | δH Hydrogen bonding |
|---|---|---|---|---|
Неполарни растварачи | ||||
| n-Hexane | CH3CH2CH2CH2CH2CH3 | 14.9 | 0.0 | 0.0 |
| Benzene | C6H6 | 18.4 | 0.0 | 2.0 |
| Toluene | C6H5-CH3 | 18.0 | 1.4 | 2.0 |
| Diethyl ether | CH3CH2-O-CH2CH3 | 14.5 | 2.9 | 4.6 |
| Chloroform | CHCl3 | 17.8 | 3.1 | 5.7 |
| 1,4-Dioxane | /-CH2-CH2-O-CH2-CH2-O-\ | 17.5 | 1.8 | 9.0 |
Поларни апротични растварачи | ||||
| Ethyl acetate | CH3-C(=O)-O-CH2-CH3 | 15.8 | 5.3 | 7.2 |
| Tetrahydrofuran (THF) | /-CH2-CH2-O-CH2-CH2-\ | 16.8 | 5.7 | 8.0 |
| Dichloromethane | CH2Cl2 | 17.0 | 7.3 | 7.1 |
| Acetone | CH3-C(=O)-CH3 | 15.5 | 10.4 | 7.0 |
| Acetonitrile (MeCN) | CH3-C≡N | 15.3 | 18.0 | 6.1 |
| Dimethylformamide (DMF) | H-C(=O)N(CH3)2 | 17.4 | 13.7 | 11.3 |
| Dimethyl sulfoxide (DMSO) | CH3-S(=O)-CH3 | 18.4 | 16.4 | 10.2 |
Поларни протични растварачи | ||||
| Acetic acid | CH3-C(=O)OH | 14.5 | 8.0 | 13.5 |
| n-Butanol | CH3CH2CH2CH2OH | 16.0 | 5.7 | 15.8 |
| Isopropanol | CH3-CH(-OH)-CH3 | 15.8 | 6.1 | 16.4 |
| n-Propanol | CH3CH2CH2OH | 16.0 | 6.8 | 17.4 |
| Ethanol | CH3CH2OH | 15.8 | 8.8 | 19.4 |
| Methanol | CH3OH | 14.7 | 12.3 | 22.3 |
| Formic acid | H-C(=O)OH | 14.6 | 10.0 | 14.0 |
| Water | H-O-H | 15.5 | 16.0 | 42.3 |
If, for environmental or other reasons, a solvent or solvent blend is required to replace another of equivalent solvency, the substitution can be made on the basis of the Hansen solubility parameters of each. The values for mixtures are taken as the weighted averages of the values for the neat solvents. This can be calculated by trial-and-error, a spreadsheet of values, or HSP software.[9][10] A 1:1 mixture of toluene and 1,4 dioxane has δD, δP and δH values of 17.8, 1.6 and 5.5, comparable to those of chloroform at 17.8, 3.1 and 5.7 respectively. Because of the health hazards associated with toluene itself, other mixtures of solvents may be found using a full HSP dataset.
Види још
Референце
- ^ Peter Atkins; Julio de Paula (2001). Physical Chemistry (7th изд.). W. H. Freeman. ISBN 0716735393.
- ^ Donald A. McQuarrie; John D. Simon (1997). Physical Chemistry: A Molecular Approach (1st изд.). University Science Books. ISBN 0935702997.
- ^ dcpt.ru Solvent 646 Characteristics (ru)
- ^ dcpt.ru Solvent 647 Characteristics (ru)
- ^ dcpt.ru Solvent 648 Characteristics (ru)
- ^ dcpt.ru Solvent 650 Characteristics (ru)
- ^ Solvent Properties – Boiling Point Архивирано 14 јун 2011 на сајту Wayback Machine. Xydatasource.com. Retrieved on 26 January 2013.
- ^ Dielectric Constant Архивирано 4 јул 2010 на сајту Wayback Machine. Macro.lsu.edu. Retrieved on 26 January 2013.
- ^ а б Abbott S, Hansen CM (2008). Hansen solubility parameters in practice. Hansen-Solubility. ISBN 978-0-9551220-2-6.
- ^ а б Hansen CM (јануар 2002). Hansen solubility parameters: a user's handbook. CRC press. ISBN 978-0-8493-7248-3.
Литература
- Lowery TH, Richardson KS (1987). Mechanism and Theory in Organic Chemistry (3rd изд.). Harper Collins Publishers. ISBN 978-0-06-364044-3.
Спољашње везе
- "European Solvents Industry Group - ESIG - ESIG European Solvents Industry Group" Solvents in Europe.
- Table and text O-Chem Lecture
- Tables Properties and toxicities of organic solvents
- CDC – Organic Solvents – NIOSH Workplace Safety and Health Topic
- EPA – Solvent Contaminated Wipes
