Heksan
| |

| |

| |
| Nazivi | |
|---|---|
| IUPAC naziv
Heksan[1]
| |
| Identifikacija | |
3D model (Jmol)
|
|
| Bajlštajn | 1730733 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.435 |
| EC broj | 203-777-6 |
| Gmelin Referenca | 1985 |
| KEGG[2] | |
| MeSH | n-hexane |
| RTECS | MN9275000 |
| UNII | |
| UN broj | 1208 |
| |
| |
| Svojstva | |
| C6H14 | |
| Molarna masa | 86,18 g·mol−1 |
| Agregatno stanje | Bezbojna tečnost |
| Miris | Petrolni |
| Gustina | 0,6548 g mL−1 |
| 9,5 mg L−1 | |
| log P | 3.764 |
| Napon pare | 17,60 kPa (na 20.0 °C) |
| kH | 7,6 nmol Pa−1 kg−1 |
| λmax | 200 nm |
| Indeks refrakcije (nD) | 1,375 |
| Viskoznost | 294 μPa s |
| Termohemija | |
| Specifični toplotni kapacitet, C | 265,2 J K−1 mol−1 |
Standardna molarna
entropija (S |
296,06 J K−1 mol−1 |
Standardna entalpija
stvaranja (ΔfH |
−199,4–−198,0 kJ mol−1 |
Std entalpija
sagorevanja (ΔcH⦵298) |
−4180–−4140 kJ mol−1 |
| Opasnosti | |
| GHS piktogrami |    
|
| GHS signalne reči | Opasnost |
| H225, H304, H315, H336, H373, H411 | |
| P210, P261, P273, P281, P301+310, P331 | |
| NFPA 704 | |
| Tačka paljenja | −26.0 °C |
| Eksplozivni limiti | 1,2–7,7% |
| Smrtonosna doza ili koncentracija (LD, LC): | |
LD50 (srednja doza)
|
25 g kg−1 (oralno, pacov) |
| Srodna jedinjenja | |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25°C [77°F], 100 kPa). | |
| Reference infokutije | |
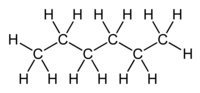
Heksan je šesti član homologog reda alkana sa molekulskom formulom CH3(CH2)4CH3. Prefiks „Heks“ označava da postoji šest atoma ugljenika, dok „an“ pokauje da pripada homologom redu alkana. Heksanovi izomeri su veoma nereaktivni.
Reference[uredi | uredi izvor]
- ^ „n-hexane – Compound Summary”. PubChem Compound. USA: National Center for Biotechnology Information. 16. 9. 2004. Identification and Related Records. Pristupljeno 31. 12. 2011.
- ^ Joanne Wixon; Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast. 17 (1): 48—55. doi:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.

