Тачка топљења — разлика између измена
Спашавам 1 извора и означавам 0 мртвим.) #IABot (v2.0.8.1 |
. ознака: везе до вишезначних одредница |
||
| Ред 1: | Ред 1: | ||
{{short description|Температура на којој чврста материја прелази у течност}} |
|||
[[Датотека:Melting ice thermometer.jpg|мини|Тачка топљења леда је 0<sup>о</sup>С]] |
|||
[[Датотека:Melting ice thermometer.jpg|мини|250п|Тачка топљења леда је 0<sup>о</sup>С]] |
|||
'''Тачка топљења''' је [[температура]] при којој [[Хемијска супстанца|супстанција]] из [[Чврсто агрегатно стање|чврстог]] [[агрегатна стања|агрегатног стања]] прелази у [[Течност|течно]].{{sfn|Hofmann|2008|pp=67}} То је карактеристична константа за сваку супстанцу. Она је обично једнака [[Тачка очвршћавања|тачки очвршћавања]]. Тачке топљења и очвршћавања добро су дефинисане само за кристалне материјале. Аморфни материјали, на пример [[стакло]], не топе се на одређеној температури већ са порастом темепратуре омекшавају. |
'''Тачка топљења''' је [[температура]] при којој [[Хемијска супстанца|супстанција]] из [[Чврсто агрегатно стање|чврстог]] [[агрегатна стања|агрегатног стања]] прелази у [[Течност|течно]].{{sfn|Hofmann|2008|pp=67}} То је карактеристична константа за сваку супстанцу. Она је обично једнака [[Тачка очвршћавања|тачки очвршћавања]]. Тачке топљења и очвршћавања добро су дефинисане само за кристалне материјале. Аморфни материјали, на пример [[стакло]], не топе се на одређеној температури већ са порастом темепратуре омекшавају. |
||
Под посебним околностима, могуће је чврсто тело загревати изнад тачке топљења без фазног прелаза у течно стање ([[суперзагревање]]), односно хладити течност испод тачке мржњења ([[суперхлађење]]). То је рецимо случај са водом на веома чистој површини од стакла. Фине [[емулзија|емулзије]] чисте воде су експериментално хлађене на -38 -{[[Степен целзијуса|°C]]}-, а да се нису заледиле. Згрушавање течности се иницира путем мале промене у особини материјала (рецимо [[вибрација]]). Ако се материја остави у потпуно мирном стању, могуће је постићи појаву суперхлађења или суперзагревања. Материје у оваквом стању су термодинамчки нестабилне и могу нагло променити агрегатно стање. Ове појаве су сличне [[хистерезис]]у код сталних [[магнет]]а. |
Под посебним околностима, могуће је чврсто тело загревати изнад тачке топљења без фазног прелаза у течно стање ([[суперзагревање]]), односно хладити течност испод тачке мржњења ([[суперхлађење]]). То је рецимо случај са водом на веома чистој површини од стакла. Фине [[емулзија|емулзије]] чисте воде су експериментално хлађене на -38 -{[[Степен целзијуса|°C]]}-, а да се нису заледиле. Згрушавање течности се иницира путем мале промене у особини материјала (рецимо [[вибрација]]). Ако се материја остави у потпуно мирном стању, могуће је постићи појаву суперхлађења или суперзагревања. Материје у оваквом стању су термодинамчки нестабилне и могу нагло променити агрегатно стање. Ове појаве су сличне [[хистерезис]]у код сталних [[магнет]]а. |
||
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the '''freezing point''' or '''crystallization point'''. Because of the ability of substances to [[Supercooling|supercool]], the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the [[#Melting point measurements|melting point]]."<ref>{{cite journal |last1=Ramsay |first1=J. A. |title=A New Method of Freezing-Point Determination for Small Quantities |journal=Journal of Experimental Biology |date=1 May 1949 |volume=26 |issue=1 |pages=57–64 |doi=10.1242/jeb.26.1.57 |pmid=15406812 |url=http://jeb.biologists.org/cgi/pmidlookup?view=long&pmid=15406812 }}</ref> |
|||
== Примери == |
|||
{{рут}} |
|||
[[File:Carboxylic.Acids.Melting.&.Boiling.Points.jpg|thumb|250п|Melting points (in blue) and boiling points (in pink) of the first eight [[carboxylic acids]] (°C)]] |
|||
For most substances, [[melting]] and [[freezing]] points are approximately equal. For example, the melting point ''and'' freezing point of [[mercury (element)|mercury]] is {{Convert|234.32|K|C F|lk=on|abbr=out}}.<ref>[[#Haynes|Haynes]], p. 4.122.</ref> However, certain substances possess differing solid-liquid transition temperatures. For example, [[agar]] melts at {{Convert|85|C|F K}} and solidifies from {{Convert|31|C|F K}}; such direction dependence is known as [[hysteresis]]. The melting point of ice at 1 atmosphere of pressure is very close<ref>The melting point of purified water has been measured as 0.002519 ± 0.000002 °C, see {{cite journal|author1=Feistel, R. |author2=Wagner, W. |year = 2006 |
|||
|title = A New Equation of State for H<sub>2</sub>O Ice Ih |
|||
|journal = J. Phys. Chem. Ref. Data|volume = 35|issue = 2 |
|||
|pages = 1021–1047 |
|||
|doi = 10.1063/1.2183324|bibcode = 2006JPCRD..35.1021F }} |
|||
</ref> to {{Convert|0|C|F K}}; this is also known as the ice point. In the presence of [[Nucleation|nucleating substances]], the freezing point of water is not always the same as the melting point. In the absence of nucleators water can exist as a [[Supercooling|supercooled]] liquid down to {{Convert|-48.3|C|F K}} before freezing. |
|||
The metal with the highest melting point is [[tungsten]], at {{Convert|3414|C|F K}};<ref>[[#Haynes|Haynes]], p. 4.123.</ref> this property makes tungsten excellent for use as [[electrical filament]]s in [[incandescent lamp]]s. The often-cited [[carbon]] does not melt at ambient pressure but [[sublimation (physics)|sublimes]] at about {{Convert|3700|C|F K|sigfig=2}}; a liquid phase only exists above pressures of {{Convert|10|MPa|atm|abbr=on}} and estimated {{Convert|4030-4430|C|F K}} (see [[:File:Carbon basic phase diagram.png|carbon phase diagram]]). [[Tantalum hafnium carbide]] (Ta<sub>4</sub>HfC<sub>5</sub>) is a [[refractory]] compound with a very high melting point of {{Convert|4215|K|C F}}.<ref>{{cite journal|title=Researches on Systems with Carbides at High Melting Point and Contributions to the Problem of Carbon Fusion|journal=Z. Tech. Phys.|author1=Agte, C. |author2=Alterthum, H. | volume= 11|year= 1930|pages=182–191}}</ref> Quantum mechanical computer simulations have predicted that the alloy HfN<sub>0.38</sub>C<sub>0.51</sub> will have an even higher melting point (about 4400 K),<ref>{{cite journal|author1= Hong, Q.-J. |author2=van de Walle, A. |year = 2015 | title = Prediction of the material with highest known melting point from ab initio molecular dynamics calculations | journal = Phys. Rev. B | volume = 92 |issue = 2 | pages = 020104(R) | doi = 10.1103/PhysRevB.92.020104 |bibcode=2015PhRvB..92b0104H |doi-access = free }}</ref> which would make it the substance with the highest melting point at ambient pressure. This prediction was later confirmed by experiment.<ref>{{cite journal |last1=Buinevich |first1=V.S. |last2=Nepapushev |first2=A.A. |last3=Moskovskikh |first3=D.O. |last4=Trusov |first4=G.V. |last5=Kuskov |first5=K.V. |last6=Vadchenko |first6=S.G. |last7=Rogachev |first7=A.S. |last8=Mukasyan |first8=A.S. |title=Fabrication of ultra-high-temperature nonstoichiometric hafnium carbonitride via combustion synthesis and spark plasma sintering |journal=Ceramics International |date=March 2020 |volume=46 |issue=10 |pages=16068–16073 |doi=10.1016/j.ceramint.2020.03.158 |s2cid=216437833 }}</ref> At the other end of the scale, [[helium]] does not freeze at all at normal pressure even at temperatures arbitrarily close to [[absolute zero]]; a pressure of more than twenty times normal [[atmospheric pressure]] is necessary. |
|||
{| class="wikitable sortable collapsible" |
|||
! colspan=4 |List of common chemicals |
|||
|- |
|||
!Chemical{{efn-ur|name=fn10}} |
|||
! data-sort-type="number" | [[Density]] {{nowrap|1=({{nobold|1={{sfrac|[[Gram|g]]|[[Cubic centimetre|cm<sup>3</sup>]]}}}})}} |
|||
! data-sort-type="number" | Melt {{nowrap|1=({{nobold|[[Kelvin|K]]}})}}<ref>{{cite journal|last1=Holman|first1=S. W.|last2=Lawrence|first2=R. R.|last3=Barr|first3=L.|title=Melting Points of Aluminum, Silver, Gold, Copper, and Platinum|journal=Proceedings of the American Academy of Arts and Sciences|date=1 January 1895|volume=31|pages=218–233|doi=10.2307/20020628|jstor=20020628}}</ref> |
|||
! data-sort-type="number" | [[Boiling point|Boil]] {{nowrap|1=({{nobold|K}})}} |
|||
|- |
|||
| Water @STP || 1 || {{convert|0|C|K|disp=number}} || {{convert|100|C|K|disp=number}} |
|||
|- |
|||
| [[Solder]] (Pb60Sn40) || || {{convert|183|C|K|disp=number}} || |
|||
|- |
|||
| [[Cocoa butter]] || || {{convert|34.1|C|K|disp=number}} || - |
|||
|- |
|||
| [[Paraffin wax]] || 0.9 || {{convert|37|C|K|disp=number}} || {{convert|370|C|K|disp=number}} |
|||
|- |
|||
| [[Hydrogen]] || 0.00008988 || {{sort|0014|14.01}} || 20.28 |
|||
|- |
|||
| [[Helium]] || 0.0001785 || {{sort|0.1|—}}{{efn-ur|name=fn6}} || 4.22 |
|||
|- |
|||
| [[Beryllium]] || 1.85 || {{sort|1560|1560}} || 2742 |
|||
|- |
|||
| [[Carbon]] || 2.267 || {{sort|0.1|—}}{{efn-ur|name=fn11}}<ref name=rsc/> || 4000{{efn-ur|name=fn11}}<ref name=rsc>{{cite web|url=https://www.rsc.org/periodic-table/element/6/carbon|title=Carbon|website=rsc.org}}</ref> |
|||
|- |
|||
| [[Nitrogen]] || 0.0012506 || {{sort|0063|63.15}} || 77.36 |
|||
|- |
|||
| [[Oxygen]] || 0.001429 || {{sort|0054|54.36}} || 90.20 |
|||
|- |
|||
| [[Sodium]] || 0.971 || {{sort|0371|370.87}} || 1156 |
|||
|- |
|||
| [[Magnesium]] || 1.738 || {{sort|0923|923}} || 1363 |
|||
|- |
|||
| [[Aluminium]] || 2.698 || {{sort|0933.5|933.47}} || 2792 |
|||
|- |
|||
| [[Sulfur]] || 2.067 || {{sort|0388.4|388.36}} || 717.87 |
|||
|- |
|||
| [[Chlorine]] || 0.003214 || {{sort|0171.6|171.6}} || 239.11 |
|||
|- |
|||
| [[Potassium]] || 0.862 || {{sort|0336.5|336.53}} || 1032 |
|||
|- |
|||
| [[Titanium]] || 4.54 || {{sort|1941|1941}} || 3560 |
|||
|- |
|||
| [[Iron]] || 7.874 || {{sort|1811|1811}} || 3134 |
|||
|- |
|||
| [[Nickel]] || 8.912 || {{sort|1728|1728}} || 3186 |
|||
|- |
|||
| [[Copper]] || 8.96 || 1357.77 || 2835 |
|||
|- |
|||
| [[Zinc]] || 7.134 || {{sort|0693|692.88}} || 1180 |
|||
|- |
|||
| [[Gallium]] || 5.907 || {{sort|0302.9|302.9146}} || 2673 |
|||
|- |
|||
| [[Silver]] || 10.501 || 1234.93 || 2435 |
|||
|- |
|||
| [[Cadmium]] || 8.69 || {{sort|0594.22|594.22}} || 1040 |
|||
|- |
|||
| [[Indium]] || 7.31 || {{sort|0429.75|429.75}} || 2345 |
|||
|- |
|||
| [[Iodine]] || 4.93 || {{sort|0386.85|386.85}} || 457.4 |
|||
|- |
|||
| [[Tantalum]] || 16.654 || 3290 || 5731 |
|||
|- |
|||
| [[Tungsten]] || 19.25 || 3695 || 5828 |
|||
|- |
|||
| [[Platinum]] || 21.46 || 2041.4 || 4098 |
|||
|- |
|||
| [[Gold]] || 19.282 || 1337.33 || 3129 |
|||
|- |
|||
| [[Mercury (element)|Mercury]] || 13.5336 || {{sort|0234.43|234.43}} || 629.88 |
|||
|- |
|||
| [[Lead]] || 11.342 || {{sort|0600.61|600.61}} || 2022 |
|||
|- |
|||
| [[Bismuth]] || 9.807 || {{sort|0544.7|544.7}} || 1837 |
|||
|- |
|||
|- class="sortbottom" |
|||
| colspan=13 |<div class="mw-collapsible mw-collapsed"> |
|||
Notes<!-- Footnotes are defined here --> |
|||
<div class="mw-collapsible-content">{{notelist|group=upper-roman|refs= |
|||
{{efn-ur|name=fn6|Helium does not solidify at a pressure of one atmosphere. Helium can only solidify at pressures above 25 atmospheres, which corresponds to a melting point of absolute zero.}} |
|||
{{efn-ur|name=fn10|Z is the standard symbol for [[atomic number]]; C is the standard symbol for [[heat capacity]]; and χ is the standard symbol for [[electronegativity]] on the Pauling scale.}} |
|||
{{efn-ur|name=fn11|Carbon does not melt at any temperature under standard pressure, instead it sublimes around 4100K}} |
|||
}}</div></div> |
|||
|} |
|||
== Термодинамика == |
|||
[[Датотека:Melting curve of water.svg|thumb|250п|Pressure dependence of water melting point.]] |
|||
[[Датотека:Si(tms)4.png|thumb|right|250px|Like many high symmetry compounds, [[tetrakis(trimethylsilyl)silane]] has a very high melting point (m.p.) of 319-321 °C. It tends to sublime, so the m.p. determination requires that the sample be sealed in a tube.<ref name=Gilman>{{cite journal|title= Tetrakis(trimethylsilyl)silane|author1=Gilman, H. |author2=Smith, C. L. |journal=Journal of Organometallic Chemistry|year=1967|volume=8|issue=2 |pages=245–253|doi=10.1016/S0022-328X(00)91037-4}}</ref>]] |
|||
For a solid to melt, heat is required to raise its temperature to the melting point. However, further heat needs to be supplied for the melting to take place: this is called the [[heat of fusion]], and is an example of [[latent heat]]. |
|||
From a thermodynamics point of view, at the melting point the change in [[Gibbs free energy]] (ΔG) of the material is zero, but the [[enthalpy]] (''H'') and the [[entropy]] (''S'') of the material are increasing (ΔH, ΔS > 0). Melting phenomenon happens when the Gibbs free energy of the liquid becomes lower than the solid for that material. At various pressures this happens at a specific temperature. It can also be shown that: |
|||
: <math>\Delta S = \frac {\Delta H} {T}</math> |
|||
Here ''T'', ''ΔS'' and ''ΔH'' are respectively the [[temperature]] at the melting point, change of entropy of melting and the change of enthalpy of melting. |
|||
The melting point is sensitive to extremely large changes in [[pressure]], but generally this sensitivity is orders of magnitude less than that for the [[boiling point]], because the solid-liquid transition represents only a small change in volume.<ref>The exact relationship is expressed in the [[Clausius–Clapeyron relation]].</ref><ref>{{cite web |url= http://mpec.sc.mahidol.ac.th/RADOK/physmath/PHYSICS/j10.htm |title= J10 Heat: Change of aggregate state of substances through change of heat content: Change of aggregate state of substances and the equation of Clapeyron-Clausius |access-date= 19 February 2008}}</ref> If, as observed in most cases, a substance is more dense in the solid than in the liquid state, the melting point will increase with increases in pressure. Otherwise the reverse behavior occurs. Notably, this is the case of water, as illustrated graphically to the right, but also of Si, Ge, Ga, Bi. With extremely large changes in pressure, substantial changes to the melting point are observed. For example, the melting point of silicon at ambient pressure (0.1 MPa) is 1415 °C, but at pressures in excess of 10 GPa it decreases to 1000 °C.<ref>Tonkov, E. Yu. and Ponyatovsky, E. G. (2005) ''Phase Transformations of Elements Under High Pressure'', CRC Press, Boca Raton, p. 98 {{ISBN|0-8493-3367-9}}</ref> |
|||
== Карнелијево правило == |
|||
In [[organic chemistry]], '''Carnelley's rule''', established in 1882 by [[Thomas Carnelley]], states that ''high [[molecular symmetry]] is associated with high melting point''.<ref>{{cite journal |title= Melting Point and Molecular Symmetry |journal= [[Journal of Chemical Education]] |page= 724 |volume= 77 |issue= 6 |year= 2000 |doi= 10.1021/ed077p724 |author1=Brown, R. J. C. |author2=R. F. C. |bibcode = 2000JChEd..77..724B }}</ref> Carnelley based his rule on examination of 15,000 chemical compounds. For example, for three [[structural isomer]]s with [[molecular formula]] C<sub>5</sub>H<sub>12</sub> the melting point increases in the series [[isopentane]] −160 °C (113 K) [[n-pentane]] −129.8 °C (143 K) and [[neopentane]] −16.4 °C (256.8 K).<ref>[[#Haynes|Haynes]], pp. 6.153–155.</ref> Likewise in [[xylene]]s and also [[dichlorobenzene]]s the melting point increases in the order [[arene substitution patterns|meta, ortho and then para]]. [[Pyridine]] has a lower symmetry than [[benzene]] hence its lower melting point but the melting point again increases with [[diazine]] and [[triazine]]s. Many cage-like compounds like [[adamantane]] and [[cubane]] with high symmetry have relatively high melting points. |
|||
A high melting point results from a high [[heat of fusion]], a low [[entropy of fusion]], or a combination of both. In highly symmetrical molecules the crystal phase is densely packed with many efficient intermolecular interactions resulting in a higher enthalpy change on melting. |
|||
== Предвиђање тачке топљења == |
|||
In February 2011, [[Alfa Aesar]] released over 10,000 melting points of compounds from their catalog as [[open data]]. This dataset has been used to create a [[random forest]] model for melting point prediction which is now freely available.<ref name=LangGuha>[http://www.qsardb.org/repository/predictor/10967/104?model=rf Predict melting point from SMILES]. Qsardb.org. Retrieved on 13 September 2013.</ref> Open melting point data are also available from ''[[Nature Precedings]]''.<ref name=Williams>{{cite journal |last1=Bradley |first1=Jean-Claude |last2=Lang |first2=Andrew |last3=Williams |first3=Antony |last4=Curtin |first4=Evan |title=ONS Open Melting Point Collection |journal=Nature Precedings |date=11 August 2011 |doi=10.1038/npre.2011.6229.1 |doi-access=free }}</ref> High quality data mined from patents and also models<ref name=OCHEM>[http://ochem.eu/article/99826 OCHEM melting point models]. ochem.eu. Retrieved on 18 June 2016.</ref> developed with these data were published by Tetko ''et al''.<ref name=Tetko>{{cite journal | doi = 10.1186/s13321-016-0113-y | title = The development of models to predict melting and pyrolysis point data associated with several hundred thousand compounds mined from PATENTS | journal = Journal of Cheminformatics | volume = 8 | year = 2016 | last1 = Tetko | first1 = Igor V | last2 = m. Lowe | first2 = Daniel | last3 = Williams | first3 = Antony J | pages = 2 | pmc = 4724158 | pmid=26807157}}</ref> |
|||
== Види још == |
== Види још == |
||
| Ред 12: | Ред 135: | ||
== Литература == |
== Литература == |
||
{{refbegin}} |
|||
* {{cite book |ref = Haynes |editor-last = Haynes |editor-first = William M. |year = 2011 |title = CRC Handbook of Chemistry and Physics |edition = 92nd |publisher = CRC Press |isbn = 978-1439855119 }} |
|||
* {{Cite book|ref=harv|last=Hofmann|first=Philip|title=Solid state physics: an introduction|url=http://books.google.com/books?id=XfIpxi4kcM4C&pg=PA67|accessdate=13. 3. 2011.|year=2008|publisher=Wiley-VCH|isbn=978-3-527-40861-0|archive-date=21. 03. 2015|archive-url=https://web.archive.org/web/20150321072209/http://books.google.com/books?id=XfIpxi4kcM4C&pg=PA67|url-status=dead}} |
* {{Cite book|ref=harv|last=Hofmann|first=Philip|title=Solid state physics: an introduction|url=http://books.google.com/books?id=XfIpxi4kcM4C&pg=PA67|accessdate=13. 3. 2011.|year=2008|publisher=Wiley-VCH|isbn=978-3-527-40861-0|archive-date=21. 03. 2015|archive-url=https://web.archive.org/web/20150321072209/http://books.google.com/books?id=XfIpxi4kcM4C&pg=PA67|url-status=dead}} |
||
{{refend}} |
|||
== Спољашње везе == |
|||
{{Commons category|Melting point}} |
|||
* [https://archive.org/details/meltingboilingpo01carnuoft Melting and boiling point tables vol. 1] by Thomas Carnelley (Harrison, London, 1885–1887) |
|||
* [https://archive.org/details/meltingboilingpo02carnuoft Melting and boiling point tables vol. 2] by Thomas Carnelley (Harrison, London, 1885–1887) |
|||
* [http://ochem.eu/article/99826 Patent mined data] Over 250,000 freely downloadable melting point data. Also downloadable at [https://figshare.com/articles/Melt-ing_Point_and_Pyrolysis_Point_Data_for_Tens_of_Thousands_of_Chemicals/2007426 figshare] |
|||
{{Authority control}} |
|||
[[Категорија:Температура]] |
[[Категорија:Температура]] |
||
Верзија на датум 27. новембар 2021. у 09:20

Тачка топљења је температура при којој супстанција из чврстог агрегатног стања прелази у течно.[1] То је карактеристична константа за сваку супстанцу. Она је обично једнака тачки очвршћавања. Тачке топљења и очвршћавања добро су дефинисане само за кристалне материјале. Аморфни материјали, на пример стакло, не топе се на одређеној температури већ са порастом темепратуре омекшавају.
Под посебним околностима, могуће је чврсто тело загревати изнад тачке топљења без фазног прелаза у течно стање (суперзагревање), односно хладити течност испод тачке мржњења (суперхлађење). То је рецимо случај са водом на веома чистој површини од стакла. Фине емулзије чисте воде су експериментално хлађене на -38 °C, а да се нису заледиле. Згрушавање течности се иницира путем мале промене у особини материјала (рецимо вибрација). Ако се материја остави у потпуно мирном стању, могуће је постићи појаву суперхлађења или суперзагревања. Материје у оваквом стању су термодинамчки нестабилне и могу нагло променити агрегатно стање. Ове појаве су сличне хистерезису код сталних магнета.
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."[2]
Примери
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
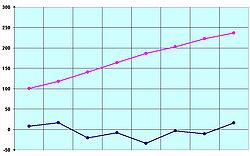
For most substances, melting and freezing points are approximately equal. For example, the melting point and freezing point of mercury is 23.432 kelvins (23.159 °C; 41.718 °F).[3] However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F; 358 K) and solidifies from 31 °C (88 °F; 304 K); such direction dependence is known as hysteresis. The melting point of ice at 1 atmosphere of pressure is very close[4] to 0 °C (32 °F; 273 K); this is also known as the ice point. In the presence of nucleating substances, the freezing point of water is not always the same as the melting point. In the absence of nucleators water can exist as a supercooled liquid down to −483 °C (−837 °F; −210 K) before freezing.
The metal with the highest melting point is tungsten, at 3.414 °C (6.177 °F; 3.687 K);[5] this property makes tungsten excellent for use as electrical filaments in incandescent lamps. The often-cited carbon does not melt at ambient pressure but sublimes at about 3.700 °C (6.700 °F; 4.000 K); a liquid phase only exists above pressures of 10 MPa (99 atm) and estimated 4.030—4.430 °C (7.290—8.010 °F; 4.300—4.700 K) (see carbon phase diagram). Tantalum hafnium carbide (Ta4HfC5) is a refractory compound with a very high melting point of 4.215 K (3.942 °C; 7.127 °F).[6] Quantum mechanical computer simulations have predicted that the alloy HfN0.38C0.51 will have an even higher melting point (about 4400 K),[7] which would make it the substance with the highest melting point at ambient pressure. This prediction was later confirmed by experiment.[8] At the other end of the scale, helium does not freeze at all at normal pressure even at temperatures arbitrarily close to absolute zero; a pressure of more than twenty times normal atmospheric pressure is necessary.
| List of common chemicals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical[I] | Density (g/cm3) | Melt (K)[9] | Boil (K) | |||||||||
| Water @STP | 1 | 273 | 373 | |||||||||
| Solder (Pb60Sn40) | 456 | |||||||||||
| Cocoa butter | 614 | - | ||||||||||
| Paraffin wax | 0.9 | 310 | 643 | |||||||||
| Hydrogen | 0.00008988 | 14.01 | 20.28 | |||||||||
| Helium | 0.0001785 | —[II] | 4.22 | |||||||||
| Beryllium | 1.85 | 1560 | 2742 | |||||||||
| Carbon | 2.267 | —[III][10] | 4000[III][10] | |||||||||
| Nitrogen | 0.0012506 | 63.15 | 77.36 | |||||||||
| Oxygen | 0.001429 | 54.36 | 90.20 | |||||||||
| Sodium | 0.971 | 370.87 | 1156 | |||||||||
| Magnesium | 1.738 | 923 | 1363 | |||||||||
| Aluminium | 2.698 | 933.47 | 2792 | |||||||||
| Sulfur | 2.067 | 388.36 | 717.87 | |||||||||
| Chlorine | 0.003214 | 171.6 | 239.11 | |||||||||
| Potassium | 0.862 | 336.53 | 1032 | |||||||||
| Titanium | 4.54 | 1941 | 3560 | |||||||||
| Iron | 7.874 | 1811 | 3134 | |||||||||
| Nickel | 8.912 | 1728 | 3186 | |||||||||
| Copper | 8.96 | 1357.77 | 2835 | |||||||||
| Zinc | 7.134 | 692.88 | 1180 | |||||||||
| Gallium | 5.907 | 302.9146 | 2673 | |||||||||
| Silver | 10.501 | 1234.93 | 2435 | |||||||||
| Cadmium | 8.69 | 594.22 | 1040 | |||||||||
| Indium | 7.31 | 429.75 | 2345 | |||||||||
| Iodine | 4.93 | 386.85 | 457.4 | |||||||||
| Tantalum | 16.654 | 3290 | 5731 | |||||||||
| Tungsten | 19.25 | 3695 | 5828 | |||||||||
| Platinum | 21.46 | 2041.4 | 4098 | |||||||||
| Gold | 19.282 | 1337.33 | 3129 | |||||||||
| Mercury | 13.5336 | 234.43 | 629.88 | |||||||||
| Lead | 11.342 | 600.61 | 2022 | |||||||||
| Bismuth | 9.807 | 544.7 | 1837 | |||||||||
Notes
| ||||||||||||
Термодинамика


For a solid to melt, heat is required to raise its temperature to the melting point. However, further heat needs to be supplied for the melting to take place: this is called the heat of fusion, and is an example of latent heat.
From a thermodynamics point of view, at the melting point the change in Gibbs free energy (ΔG) of the material is zero, but the enthalpy (H) and the entropy (S) of the material are increasing (ΔH, ΔS > 0). Melting phenomenon happens when the Gibbs free energy of the liquid becomes lower than the solid for that material. At various pressures this happens at a specific temperature. It can also be shown that:
Here T, ΔS and ΔH are respectively the temperature at the melting point, change of entropy of melting and the change of enthalpy of melting.
The melting point is sensitive to extremely large changes in pressure, but generally this sensitivity is orders of magnitude less than that for the boiling point, because the solid-liquid transition represents only a small change in volume.[12][13] If, as observed in most cases, a substance is more dense in the solid than in the liquid state, the melting point will increase with increases in pressure. Otherwise the reverse behavior occurs. Notably, this is the case of water, as illustrated graphically to the right, but also of Si, Ge, Ga, Bi. With extremely large changes in pressure, substantial changes to the melting point are observed. For example, the melting point of silicon at ambient pressure (0.1 MPa) is 1415 °C, but at pressures in excess of 10 GPa it decreases to 1000 °C.[14]
Карнелијево правило
In organic chemistry, Carnelley's rule, established in 1882 by Thomas Carnelley, states that high molecular symmetry is associated with high melting point.[15] Carnelley based his rule on examination of 15,000 chemical compounds. For example, for three structural isomers with molecular formula C5H12 the melting point increases in the series isopentane −160 °C (113 K) n-pentane −129.8 °C (143 K) and neopentane −16.4 °C (256.8 K).[16] Likewise in xylenes and also dichlorobenzenes the melting point increases in the order meta, ortho and then para. Pyridine has a lower symmetry than benzene hence its lower melting point but the melting point again increases with diazine and triazines. Many cage-like compounds like adamantane and cubane with high symmetry have relatively high melting points.
A high melting point results from a high heat of fusion, a low entropy of fusion, or a combination of both. In highly symmetrical molecules the crystal phase is densely packed with many efficient intermolecular interactions resulting in a higher enthalpy change on melting.
Предвиђање тачке топљења
In February 2011, Alfa Aesar released over 10,000 melting points of compounds from their catalog as open data. This dataset has been used to create a random forest model for melting point prediction which is now freely available.[17] Open melting point data are also available from Nature Precedings.[18] High quality data mined from patents and also models[19] developed with these data were published by Tetko et al.[20]
Види још
Референце
- ^ Hofmann 2008, стр. 67.
- ^ Ramsay, J. A. (1. 5. 1949). „A New Method of Freezing-Point Determination for Small Quantities”. Journal of Experimental Biology. 26 (1): 57—64. PMID 15406812. doi:10.1242/jeb.26.1.57.
- ^ Haynes, p. 4.122.
- ^ The melting point of purified water has been measured as 0.002519 ± 0.000002 °C, see Feistel, R.; Wagner, W. (2006). „A New Equation of State for H2O Ice Ih”. J. Phys. Chem. Ref. Data. 35 (2): 1021—1047. Bibcode:2006JPCRD..35.1021F. doi:10.1063/1.2183324.
- ^ Haynes, p. 4.123.
- ^ Agte, C.; Alterthum, H. (1930). „Researches on Systems with Carbides at High Melting Point and Contributions to the Problem of Carbon Fusion”. Z. Tech. Phys. 11: 182—191.
- ^ Hong, Q.-J.; van de Walle, A. (2015). „Prediction of the material with highest known melting point from ab initio molecular dynamics calculations”. Phys. Rev. B. 92 (2): 020104(R). Bibcode:2015PhRvB..92b0104H. doi:10.1103/PhysRevB.92.020104
 .
.
- ^ Buinevich, V.S.; Nepapushev, A.A.; Moskovskikh, D.O.; Trusov, G.V.; Kuskov, K.V.; Vadchenko, S.G.; Rogachev, A.S.; Mukasyan, A.S. (март 2020). „Fabrication of ultra-high-temperature nonstoichiometric hafnium carbonitride via combustion synthesis and spark plasma sintering”. Ceramics International. 46 (10): 16068—16073. S2CID 216437833. doi:10.1016/j.ceramint.2020.03.158.
- ^ Holman, S. W.; Lawrence, R. R.; Barr, L. (1. 1. 1895). „Melting Points of Aluminum, Silver, Gold, Copper, and Platinum”. Proceedings of the American Academy of Arts and Sciences. 31: 218—233. JSTOR 20020628. doi:10.2307/20020628.
- ^ а б „Carbon”. rsc.org.
- ^ Gilman, H.; Smith, C. L. (1967). „Tetrakis(trimethylsilyl)silane”. Journal of Organometallic Chemistry. 8 (2): 245—253. doi:10.1016/S0022-328X(00)91037-4.
- ^ The exact relationship is expressed in the Clausius–Clapeyron relation.
- ^ „J10 Heat: Change of aggregate state of substances through change of heat content: Change of aggregate state of substances and the equation of Clapeyron-Clausius”. Приступљено 19. 2. 2008.
- ^ Tonkov, E. Yu. and Ponyatovsky, E. G. (2005) Phase Transformations of Elements Under High Pressure, CRC Press, Boca Raton, p. 98 ISBN 0-8493-3367-9
- ^ Brown, R. J. C.; R. F. C. (2000). „Melting Point and Molecular Symmetry”. Journal of Chemical Education. 77 (6): 724. Bibcode:2000JChEd..77..724B. doi:10.1021/ed077p724.
- ^ Haynes, pp. 6.153–155.
- ^ Predict melting point from SMILES. Qsardb.org. Retrieved on 13 September 2013.
- ^ Bradley, Jean-Claude; Lang, Andrew; Williams, Antony; Curtin, Evan (11. 8. 2011). „ONS Open Melting Point Collection”. Nature Precedings. doi:10.1038/npre.2011.6229.1
 .
.
- ^ OCHEM melting point models. ochem.eu. Retrieved on 18 June 2016.
- ^ Tetko, Igor V; m. Lowe, Daniel; Williams, Antony J (2016). „The development of models to predict melting and pyrolysis point data associated with several hundred thousand compounds mined from PATENTS”. Journal of Cheminformatics. 8: 2. PMC 4724158
 . PMID 26807157. doi:10.1186/s13321-016-0113-y.
. PMID 26807157. doi:10.1186/s13321-016-0113-y.
Литература
- Haynes, William M., ур. (2011). CRC Handbook of Chemistry and Physics (92nd изд.). CRC Press. ISBN 978-1439855119.
- Hofmann, Philip (2008). Solid state physics: an introduction. Wiley-VCH. ISBN 978-3-527-40861-0. Архивирано из оригинала 21. 03. 2015. г. Приступљено 13. 3. 2011.
Спољашње везе
- Melting and boiling point tables vol. 1 by Thomas Carnelley (Harrison, London, 1885–1887)
- Melting and boiling point tables vol. 2 by Thomas Carnelley (Harrison, London, 1885–1887)
- Patent mined data Over 250,000 freely downloadable melting point data. Also downloadable at figshare

