Ожеов ефекат — разлика између измена
м исправак интерпункције и козметичке измене |
. |
||
| Ред 1: | Ред 1: | ||
{{short description|Физички феномен}}{{рут}} |
|||
| ⚫ | '''Ожеов ефекат''' (Ожеов електрон) је емисија секундарног [[електрон]]а из [[атом]]ског омотача проузрокована емисијом електрона (стимулисаном спољашњим агенсом) из стања са великом везивном енергијом.<ref>{{GoldBookRef|title=Auger effect|file=A00520}}</ref> Овај секундарни електрон, који излеће из слабије везаног стања, назива се Ожеов електрон. На његово место углавном долази сусједни електрон са већом везивном енергијом, који тај вишак енергије емитује као [[фотон]] - у пракси је познат као [[карактеристични фотон]] (јер има познату енергију). На тај начин је могуће добити и [[Рендгенски зраци|рендгенске зраке]] или [[гама зрачење]]. |
||
[[File:Auger Process.svg|thumb|340px|Two views of the Auger process. (a) illustrates sequentially the steps involved in Auger deexcitation. An incident electron (or photon) creates a core hole in the 1s level. An electron from the 2s level fills in the 1s hole and the transition energy is imparted to a 2p electron which is emitted. The final atomic state thus has two holes, one in the 2s orbital and the other in the 2p orbital. (b) illustrates the same process using [[X-ray notation]], KL<sub alt="KL_1L_{2,3}">1</sub>L<sub>2,3</sub>.]] |
|||
| ⚫ | '''Ожеов ефекат''' (Ожеов електрон) је емисија секундарног [[електрон]]а из [[атом]]ског омотача<ref>{{cite journal|title= The definition of core electrons|journal= Chemical Physics Letters|date= 2001-12-28|pages= 573–576|volume= 350|issue= 5–6|doi= 10.1016/S0009-2614(01)01345-8|first= Vitaly A|last= Rassolov|first2= John A|last2= Pople|first3= Paul C|last3= Redfern|first4= Larry A|last4= Curtiss|bibcode= 2001CPL...350..573R}}</ref> проузрокована емисијом електрона (стимулисаном спољашњим агенсом) из стања са великом везивном енергијом.<ref>{{GoldBookRef|title=Auger effect|file=A00520}}</ref> Овај секундарни електрон, који излеће из слабије везаног стања, назива се Ожеов електрон. На његово место углавном долази сусједни електрон са већом везивном енергијом, који тај вишак енергије емитује као [[фотон]] - у пракси је познат као [[карактеристични фотон]] (јер има познату енергију).<ref>{{GoldBookRef|title=Auger electron|file=A00521}}</ref> На тај начин је могуће добити и [[Рендгенски зраци|рендгенске зраке]] или [[гама зрачење]].. |
||
Ефекат и секундарни електрон име су добили по француском физичару Пјеру Ожеу ({{јез-фра|Pierre Victor Auger}}) који је појаву описао. |
Ефекат и секундарни електрон име су добили по француском физичару Пјеру Ожеу ({{јез-фра|Pierre Victor Auger}}) који је појаву описао. |
||
== Ефекат == |
|||
<!--When an [[electron]] is removed from a core level of an [[atom]], leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in a release of [[energy]]. Although sometimes this energy is released in the form of an emitted [[photon]], the energy can also be transferred to another electron, which is then ejected from the atom.--> |
|||
The effect was first discovered by [[Lise Meitner]] in 1922; [[Pierre Victor Auger]] independently discovered the effect shortly after and is credited with the discovery in most of the scientific community.<ref name="grant2003">{{cite book |last=Grant |first= John T.|year=2003 |title=Surface Analysis by Auger and X-ray Photoelectron Spectroscopy |location=Chichester |publisher=IM Publications |isbn=1-901019-04-7 |author2=David Briggs}}</ref><ref>{{Cite journal |last1=Matsakis |first1=Demetrios |last2=Coster |first2=Anthea |last3=Laster |first3=Brenda |last4=Sime |first4=Ruth |date=2019-09-01 |title=A renaming proposal: "The Auger–Meitner effect" |url=https://physicstoday.scitation.org/doi/10.1063/PT.3.4281 |journal=Physics Today |volume=72 |issue=9 |pages=10–11 |doi=10.1063/PT.3.4281 |bibcode=2019PhT....72i..10M |s2cid=202939712 |issn=0031-9228}}</ref> |
|||
Upon ejection, the [[kinetic energy]] of the Auger electron corresponds to the difference between the energy of the initial [[electronic transition]]<ref>{{Cite web |url=http://www.mpq.mpg.de/Theorygroup/CIRAC/wiki/images/8/86/Samuel.pdf |last=Deléglise |first=S. |title=Observing the quantum jumps of light |access-date=September 17, 2010 |archive-url=https://web.archive.org/web/20101107043403/http://www.mpq.mpg.de/Theorygroup/CIRAC/wiki/images/8/86/Samuel.pdf |archive-date=November 7, 2010 |url-status=dead }}</ref> into the vacancy and the [[ionization energy]]<ref>{{cite book |first1=F. Albert |last1=Cotton |author1-link=F. Albert Cotton |first2=Geoffrey |last2=Wilkinson |author2-link=Geoffrey Wilkinson |title=Advanced Inorganic Chemistry |edition=5th |publisher=John Wiley |date=1988 |page=1381 |isbn=0-471-84997-9}}</ref> for the [[electron shell]] from which the Auger electron was ejected. These energy levels depend on the type of atom and the chemical environment in which the atom was located. |
|||
[[Auger electron spectroscopy]] involves the emission of Auger electrons by bombarding a sample with either [[X-ray]]s or energetic electrons and measures the intensity of Auger electrons that result as a function of the Auger electron energy. The resulting spectra can be used to determine the identity of the emitting atoms and some information about their environment. |
|||
| ⚫ | |||
| ⚫ | [[Carrier generation and recombination#Auger recombination|Auger recombination]] is a similar Auger effect which occurs in [[semiconductor]]s. An electron and [[electron hole]] (electron-hole pair) can recombine giving up their energy to an electron in the [[conduction band]], increasing its energy. The reverse effect is known as [[impact ionization]]. |
||
The reverse effect is known as [[impact ionization]].--> |
|||
The Auger effect can impact biological molecules such as DNA. Following the K-shell ionization of the component atoms of DNA, Auger electrons are ejected leading to damage of its sugar-phosphate backbone.<ref>Akinari Yokoya & Takashi Ito (2017) Photon-induced Auger effect in biological systems: a review,International Journal of Radiation Biology, 93:8, 743–756, DOI: 10.1080/09553002.2017.1312670</ref> |
|||
== Историја == |
== Историја == |
||
Овај процес емисије електрона теоријски је предвидео Роселанд 1923.<ref>S. Rosseland, Zeitschrift für Physik 14, 173 (1923).}-</ref> а прва је открила Лиза Мајтнер ({{јез-нем|Lise Meitner}}) 1920. године и објавила 1922/3.<ref> |
Овај процес емисије електрона теоријски је предвидео Роселанд 1923.<ref>S. Rosseland, Zeitschrift für Physik 14, 173 (1923).}-</ref> а прва је открила Лиза Мајтнер ({{јез-нем|Lise Meitner}}) 1920. године и објавила 1922/3.<ref>{{cite journal|doi=10.1007/BF01326962|author=L. Meitner|title=Über die Entstehung der β-Strahl-Spektren radioaktiver Substanzen|journal=Z. Phys. |volume=9|issue=1|year=1922|pages=131–144|bibcode= 1922ZPhy....9..131M|s2cid=121637546}}</ref><ref>-{L. Meitner, Zeitschrift für Physik 17, 54 (1923).}-</ref> Касније је процес открио и Оже, објавио 1925. године и дао му своје име.<ref>-{P. Auger, Journal de Physique Radium 6, 205 (1925).</ref> |
||
The French physicist [[Pierre Victor Auger]] independently discovered it in 1923<ref>P. Auger: [http://gallica.bnf.fr/ark:/12148/bpt6k3130n.image.f187.langFR Sur les rayons β secondaires produits dans un gaz par des rayons X], C.R.A.S. 177 (1923) 169–171.</ref> upon analysis of a Wilson [[cloud chamber]] experiment and it became the central part of his PhD work.<ref>{{cite journal|doi=10.3139/146.110163|title=Pierre Auger – Lise Meitner: Comparative contributions to the Auger effect|year=2009|last1=Duparc|first1=Olivier Hardouin|journal=International Journal of Materials Research |volume=100|issue=9|pages=1162–1166|s2cid=229164774 }}</ref> High-energy X-rays were applied to ionize gas particles and observe [[photoelectric]] electrons. The observation of electron tracks that were independent of the frequency of the incident photon suggested a mechanism for electron ionization that was caused from an [[internal conversion]] of energy from a radiationless transition. Further investigation, and theoretical work using elementary quantum mechanics and transition rate/transition probability calculations, showed that the effect was a radiationless effect more than an internal conversion effect.<ref>{{cite web|title= The Auger Effect and Other Radiationless Transitions|url= http://www.cambridge.org/va/academic/subjects/physics/particle-physics-and-nuclear-physics/auger-effect-and-other-radiationless-transitions?format=PB|website= Cambridge University Press|access-date= 2015-12-11}}</ref> |
|||
== Види још == |
== Види још == |
||
| Ред 22: | Ред 30: | ||
== Литература == |
== Литература == |
||
{{refbegin|30em}} |
|||
С. Мацура, Ј. Радић-Перић, АТОМИСТИКА, Факултет за физичку хемију Универзитета у Београду/Службени лист, Београд, 2004, pp. 263. |
* С. Мацура, Ј. Радић-Перић, АТОМИСТИКА, Факултет за физичку хемију Универзитета у Београду/Службени лист, Београд, 2004, pp. 263. |
||
* {{cite book|title= Inorganic Chemistry|last= Miessler, Tarr|first= G.L.|publisher= Prentice-Hall|year= 1999}} |
|||
* {{cite web|title= Quantum Primer|url= http://www.chem1.com/acad/webtut/atomic/qprimer/#Q26|website= www.chem1.com|access-date= 2015-12-11}} |
|||
* {{Cite web|date=2013-10-02|title=Periodic Trends|url=https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends|access-date=2020-09-13|website=Chemistry LibreTexts|language=en}} |
|||
* {{cite book |last1=Miessler |first1=Gary L. |last2=Tarr |first2=Donald A. |title=Inorganic Chemistry |date=1999 |publisher=Prentice Hall |isbn=0-13-841891-8 |page=41 |edition=2nd}} |
|||
* {{cite web|url=http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Table_of_the_Elements/Ionization_Energy|title=Ionization Energy|work = ChemWiki | publisher=University of California, Davis|date=2013-10-02}} |
|||
* {{cite web| date=January 15, 2018 | title=Chapter 9: Quantum Mechanics | url=http://faculty.chem.queensu.ca/people/faculty/mombourquette/FirstYrChem/Theory/ | access-date= October 31, 2020 | website= faculty.chem.queesu.ca | language=en}} |
|||
* {{cite web |url=https://www.chem.tamu.edu/class/fyp/stone/tutorialnotefiles/fundamentals/trends.htm |title=Atomic Structure : Periodic Trends |last=Stone |first=E.G. |date=December 19, 2020v |department=Department of Chemistry |website=chem.tamu.edu |publisher=Texas A&M University |location=400 Bizzell St, College Station, TX 77843, Texas, United States |language=en |access-date=December 19, 2020}} |
|||
* {{Cite web|title=Anomalous trends in ionization energy|url=https://chemistry.stackexchange.com/questions/32363/anomalous-trends-in-ionization-energy|access-date=2020-09-20|website=Chemistry Stack Exchange}} |
|||
* {{cite book |last1=Petrucci |first1=Ralph H. |last2=Harwood |first2=William S. |last3=Herring |first3=F. Geoffrey |title=General Chemistry |date=2002 |publisher=Prentice Hall |isbn=0-13-014329-4 |page=370 |edition=8th}} |
|||
* {{Cite web|url=https://www.grandinetti.org/ionization-energy-trends|title=Ionization Energy Trends {{!}} Grandinetti Group |last=Grandinetti |first=Philip J. |date=September 8, 2019 |access-date=2020-09-13|website=www.grandinetti.org}} |
|||
* {{cite web |url=https://www.kentchemistry.com/links/PT/PTIonE.htm |title=First Ionization Energy |last=Kent |first=Mr. |website=kentchemistry.com |publisher=KentChemistry |access-date=December 6, 2020 |quote=...The addition of the second electron into an already occupied orbital introduces repulsion between the electrons, thus it is easier to remove. that is why there is a dip in the ionization energy.}} |
|||
* {{Cite web|title=Group IA|url=https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch9/alkali.php|access-date=2020-09-20|website=chemed.chem.purdue.edu}} |
|||
* {{Cite web|title=Alkali Metals|url=http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/alkmet.html|access-date=2020-09-13|website=hyperphysics.phy-astr.gsu.edu}} |
|||
* {{Cite web|title=The Alkali Metals {{!}} Introduction to Chemistry|url=https://courses.lumenlearning.com/introchem/chapter/the-alkali-metals/|access-date=2020-09-13|website=courses.lumenlearning.com}} |
|||
* {{cite web |url=https://www.lenntech.com/periodic-chart-elements/ionization-energy.htm |title=Chemical elements listed by ionization energy |author=<!--Not stated--> |date=2018 |website=lenntech.com |publisher=Lenntech BV |access-date=December 6, 2020 |quote=The elements of the periodic table sorted by ionization energy click on any element's name for further information on chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry.}} |
|||
* {{cite web |url=https://www.angelo.edu/faculty/kboudrea/periodic/trends_ionization_energy.htm |title=The Parts of the Periodic Table |last=Boudreaux |first=K.A. |date=August 13, 2020 |orig-date=July 26, 2006 |department=Department of Chemistry and Biochemistry |website=angelo.edu/faculty/kboudrea/ |publisher=Angelo State University |location=2601 W. Avenue N, San Angelo, TX 76909, Texas |language=en |access-date=December 19, 2020 |via=angelo.edu}} |
|||
* {{Cite web|date=2014-07-02|title=18.10: The Group 6A Elements|url=https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_(Zumdahl_and_Decoste)/18%3A_The_Representative_Elements/18.10%3A_The_Group_6A_Elements|access-date=2020-09-20|website=Chemistry LibreTexts|language=en}} |
|||
* {{Cite web|title=Covalent Radius for all the elements in the Periodic Table|url=https://periodictable.com/Properties/A/CovalentRadius.v.log.html|access-date=2020-09-13|website=periodictable.com}} |
|||
* {{cite web |url=https://chemistry.stackexchange.com/questions/41706/why-is-ionisation-energy-of-bismuth-lower-than-lead |title=Why is ionisation energy of bismuth lower than lead? |author=Sikander |date=December 5, 2015 |website=chemistry.stackexchange.com |publisher=Chemistry Stack Exchange |access-date=December 5, 2020 |quote=Why is ionisation enthalpy of Bismuth less than that of Lead for it just comes after the latter in periodic table?}} |
|||
* {{cite web |url=https://www.chemistryworld.com/opinion/the-group-3-dilemma/3007080.article |title=The group 3 dilemma |last=Ball |first=Philip |date=April 21, 2017 |website=chemistryworld.com |publisher=Chemistry World |location=Burlington House, Piccadilly, London |language=en |access-date=December 18, 2020 |via=Royal Society of Chemistry}} |
|||
* {{cite web |url=https://phys.org/news/2015-04-ionization-potential-lawrencium-reignites-debate.html |title=Measurement of first ionization potential of lawrencium reignites debate over periodic table |last=Yirka |first=Bob |date=April 9, 2015 |department=General Physics |website=phys.org |publisher=Phys Org |agency=Phys.org |language=en |access-date= December 13, 2020 |quote=Lawrencium, at this time, appears to have a dumb-bell shape. These new findings create conflicting views on where the element should be placed on the table and has reignited debate on the way the table is structured in general.}} |
|||
* {{cite web |url=https://www.ionicviper.org/system/files/Scerri%20Parsons%20March%204th%202017%20%2B%20new%20part_0.docx |title=What elements belong in group 3 of the periodic table? |last1=Scerri |first1=Eric R. |last2=Parsons | first2=William |date= March 2017|website=www.ionicviper.org|publisher=Ionic Viper |access-date= December 7, 2020 |quote=The question of precisely which elements should be placed in group 3 of the periodic table has been debated from time to time with apparently no resolution up to this point.}} |
|||
* {{cite journal |last1=Sato |first1=T. K. |last2=Asai |first2=M. |last3=Borschevsky |first3=A. |last4=Stora |first4=T. |last5=Sato |first5=N. |last6=Kaneya |first6=Y. |last7=Tsukada |first7=K. |last8=Düllmann |first8=Ch E. |last9=Eberhardt |first9=K. |last10=Eliav |first10=E. |last11=Ichikawa |first11=S. |last12=Kaldor |first12=U. |last13=Kratz |first13=J. V. |last14=Miyashita |first14=S. |last15=Nagame |first15=Y. |last16=Ooe |first16=K. |last17=Osa |first17=A. |last18=Renisch |first18=D. |last19=Runke |first19=J. |last20=Schädel |first20=M. |last21=Thörle-Pospiech |first21=P. |last22=Toyoshima |first22=A. |last23=Trautmann |first23=N. |title=Measurement of the first ionization potential of lawrencium, element 103 |journal=Nature |date=April 2015 |volume=520 |issue=7546 |pages=209–211 |doi=10.1038/nature14342 |pmid=25855457 |bibcode=2015Natur.520..209S |s2cid=4384213 |url=http://cds.cern.ch/record/2008656 }} |
|||
* {{cite book |last1=Singh |first1=Jasvinder |chapter=Inert Gases |page=122 |chapter-url=https://books.google.com/books?id=eKnrhryjqn0C&pg=PA122 |title=Sterling Dictionary of Physics |date=1999 |publisher=Sterling Publishers Pvt. Ltd |isbn=978-81-7359-124-2 }} |
|||
* {{cite book |doi=10.1016/B978-0-7506-3365-9.50028-6 |chapter=Vanadium, Niobium and Tantalum |title=Chemistry of the Elements |year=1997 |pages=976–1001 |isbn=978-0-7506-3365-9 }} |
|||
* {{cite book |last1=Housecroft |first1=C.E. |last2=Sharpe |first2=A.G. |date=November 1, 1993 |title=Inorganic Chemistry |url=https://www.pearson.com/us/higher-education/program/Housecroft-Inorganic-Chemistry-5th-Edition/PGM2178749.html |type=eBook |series=Inorganic Chemistry |language=en |volume=3 |edition=15th |location=Switzerland |publisher=Pearson Prentice-Hall |publication-date=November 1, 1993 |pages=536, 649, 743 |doi=10.1021/ed070pA304.1|isbn=978-0-273-74275-3 |archive-url=https://archive.org/details/Inorganic_Chemistry_4th_edition_by_Catherine_Housecroft_Alan_G._Sharpe |archive-date=December 16, 2015 |access-date=December 14, 2020 }} |
|||
* {{cite journal |doi=10.1021/ed062pA137.1 |title=Modern Inorganic Chemistry (Jolly, William L.) |year=1985 |last1=Billo |first1=E. J. |journal=Journal of Chemical Education |volume=62 |issue=4 |pages=A137 |bibcode=1985JChEd..62..137B |doi-access=free }} |
|||
{{refend}} |
|||
== Спољашње везе == |
== Спољашње везе == |
||
{{Commonscat|Auger emission}} |
{{Commonscat|Auger emission}} |
||
* {{Cite web|last=Mahan|first=Bruce H.|date=1962|title=Ionization Energy|url=https://archive.org/details/ionization_energy|access-date=2020-09-13|publisher=College of Chemistry, University of California Berkeley}} |
|||
* {{Cite web|title=Monatomic Gas - an overview {{!}} ScienceDirect Topics|url=https://www.sciencedirect.com/topics/mathematics/monatomic-gas|access-date=2022-01-08|website=www.sciencedirect.com}} |
|||
{{нормативна контрола}} |
{{нормативна контрола}} |
||
Верзија на датум 10. јул 2022. у 04:52
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
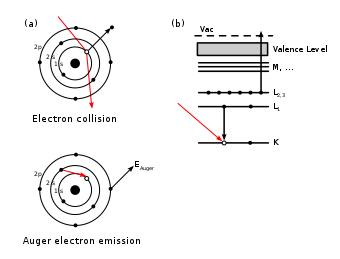
Ожеов ефекат (Ожеов електрон) је емисија секундарног електрона из атомског омотача[1] проузрокована емисијом електрона (стимулисаном спољашњим агенсом) из стања са великом везивном енергијом.[2] Овај секундарни електрон, који излеће из слабије везаног стања, назива се Ожеов електрон. На његово место углавном долази сусједни електрон са већом везивном енергијом, који тај вишак енергије емитује као фотон - у пракси је познат као карактеристични фотон (јер има познату енергију).[3] На тај начин је могуће добити и рендгенске зраке или гама зрачење..
Ефекат и секундарни електрон име су добили по француском физичару Пјеру Ожеу (франц. Pierre Victor Auger) који је појаву описао.
Ефекат
The effect was first discovered by Lise Meitner in 1922; Pierre Victor Auger independently discovered the effect shortly after and is credited with the discovery in most of the scientific community.[4][5]
Upon ejection, the kinetic energy of the Auger electron corresponds to the difference between the energy of the initial electronic transition[6] into the vacancy and the ionization energy[7] for the electron shell from which the Auger electron was ejected. These energy levels depend on the type of atom and the chemical environment in which the atom was located.
Auger electron spectroscopy involves the emission of Auger electrons by bombarding a sample with either X-rays or energetic electrons and measures the intensity of Auger electrons that result as a function of the Auger electron energy. The resulting spectra can be used to determine the identity of the emitting atoms and some information about their environment.
Auger recombination is a similar Auger effect which occurs in semiconductors. An electron and electron hole (electron-hole pair) can recombine giving up their energy to an electron in the conduction band, increasing its energy. The reverse effect is known as impact ionization.
The Auger effect can impact biological molecules such as DNA. Following the K-shell ionization of the component atoms of DNA, Auger electrons are ejected leading to damage of its sugar-phosphate backbone.[8]
Историја
Овај процес емисије електрона теоријски је предвидео Роселанд 1923.[9] а прва је открила Лиза Мајтнер (нем. Lise Meitner) 1920. године и објавила 1922/3.[10][11] Касније је процес открио и Оже, објавио 1925. године и дао му своје име.[12]
The French physicist Pierre Victor Auger independently discovered it in 1923[13] upon analysis of a Wilson cloud chamber experiment and it became the central part of his PhD work.[14] High-energy X-rays were applied to ionize gas particles and observe photoelectric electrons. The observation of electron tracks that were independent of the frequency of the incident photon suggested a mechanism for electron ionization that was caused from an internal conversion of energy from a radiationless transition. Further investigation, and theoretical work using elementary quantum mechanics and transition rate/transition probability calculations, showed that the effect was a radiationless effect more than an internal conversion effect.[15]
Види још
Референце
- ^ Rassolov, Vitaly A; Pople, John A; Redfern, Paul C; Curtiss, Larry A (2001-12-28). „The definition of core electrons”. Chemical Physics Letters. 350 (5–6): 573—576. Bibcode:2001CPL...350..573R. doi:10.1016/S0009-2614(01)01345-8.
- ^ IUPAC. „Auger effect”. Kompendijum hemijske terminologije (Internet izdanje).
- ^ IUPAC. „Auger electron”. Kompendijum hemijske terminologije (Internet izdanje).
- ^ Grant, John T.; David Briggs (2003). Surface Analysis by Auger and X-ray Photoelectron Spectroscopy. Chichester: IM Publications. ISBN 1-901019-04-7.
- ^ Matsakis, Demetrios; Coster, Anthea; Laster, Brenda; Sime, Ruth (2019-09-01). „A renaming proposal: "The Auger–Meitner effect"”. Physics Today. 72 (9): 10—11. Bibcode:2019PhT....72i..10M. ISSN 0031-9228. S2CID 202939712. doi:10.1063/PT.3.4281.
- ^ Deléglise, S. „Observing the quantum jumps of light” (PDF). Архивирано из оригинала (PDF) 7. 11. 2010. г. Приступљено 17. 9. 2010.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey (1988). Advanced Inorganic Chemistry (5th изд.). John Wiley. стр. 1381. ISBN 0-471-84997-9.
- ^ Akinari Yokoya & Takashi Ito (2017) Photon-induced Auger effect in biological systems: a review,International Journal of Radiation Biology, 93:8, 743–756, DOI: 10.1080/09553002.2017.1312670
- ^ S. Rosseland, Zeitschrift für Physik 14, 173 (1923).}-
- ^ L. Meitner (1922). „Über die Entstehung der β-Strahl-Spektren radioaktiver Substanzen”. Z. Phys. 9 (1): 131—144. Bibcode:1922ZPhy....9..131M. S2CID 121637546. doi:10.1007/BF01326962.
- ^ L. Meitner, Zeitschrift für Physik 17, 54 (1923).
- ^ -{P. Auger, Journal de Physique Radium 6, 205 (1925).
- ^ P. Auger: Sur les rayons β secondaires produits dans un gaz par des rayons X, C.R.A.S. 177 (1923) 169–171.
- ^ Duparc, Olivier Hardouin (2009). „Pierre Auger – Lise Meitner: Comparative contributions to the Auger effect”. International Journal of Materials Research. 100 (9): 1162—1166. S2CID 229164774. doi:10.3139/146.110163.
- ^ „The Auger Effect and Other Radiationless Transitions”. Cambridge University Press. Приступљено 2015-12-11.
Литература
- С. Мацура, Ј. Радић-Перић, АТОМИСТИКА, Факултет за физичку хемију Универзитета у Београду/Службени лист, Београд, 2004, pp. 263.
- Miessler, Tarr, G.L. (1999). Inorganic Chemistry. Prentice-Hall.
- „Quantum Primer”. www.chem1.com. Приступљено 2015-12-11.
- „Periodic Trends”. Chemistry LibreTexts (на језику: енглески). 2013-10-02. Приступљено 2020-09-13.
- Miessler, Gary L.; Tarr, Donald A. (1999). Inorganic Chemistry (2nd изд.). Prentice Hall. стр. 41. ISBN 0-13-841891-8.
- „Ionization Energy”. ChemWiki. University of California, Davis. 2013-10-02.
- „Chapter 9: Quantum Mechanics”. faculty.chem.queesu.ca (на језику: енглески). 15. 1. 2018. Приступљено 31. 10. 2020.
- Stone, E.G. (19. 12. 2020v). „Atomic Structure : Periodic Trends”. Department of Chemistry. chem.tamu.edu (на језику: енглески). 400 Bizzell St, College Station, TX 77843, Texas, United States: Texas A&M University. Приступљено 19. 12. 2020.
- „Anomalous trends in ionization energy”. Chemistry Stack Exchange. Приступљено 2020-09-20.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th изд.). Prentice Hall. стр. 370. ISBN 0-13-014329-4.
- Grandinetti, Philip J. (8. 9. 2019). „Ionization Energy Trends | Grandinetti Group”. www.grandinetti.org. Приступљено 2020-09-13.
- Kent, Mr. „First Ionization Energy”. kentchemistry.com. KentChemistry. Приступљено 6. 12. 2020. „...The addition of the second electron into an already occupied orbital introduces repulsion between the electrons, thus it is easier to remove. that is why there is a dip in the ionization energy.”
- „Group IA”. chemed.chem.purdue.edu. Приступљено 2020-09-20.
- „Alkali Metals”. hyperphysics.phy-astr.gsu.edu. Приступљено 2020-09-13.
- „The Alkali Metals | Introduction to Chemistry”. courses.lumenlearning.com. Приступљено 2020-09-13.
- „Chemical elements listed by ionization energy”. lenntech.com. Lenntech BV. 2018. Приступљено 6. 12. 2020. „The elements of the periodic table sorted by ionization energy click on any element's name for further information on chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry.”
- Boudreaux, K.A. (13. 8. 2020). „The Parts of the Periodic Table”. Department of Chemistry and Biochemistry. angelo.edu/faculty/kboudrea/ (на језику: енглески). 2601 W. Avenue N, San Angelo, TX 76909, Texas: Angelo State University. Приступљено 19. 12. 2020 — преко angelo.edu. Непознати параметар
|orig-date=игнорисан (помоћ) - „18.10: The Group 6A Elements”. Chemistry LibreTexts (на језику: енглески). 2014-07-02. Приступљено 2020-09-20.
- „Covalent Radius for all the elements in the Periodic Table”. periodictable.com. Приступљено 2020-09-13.
- Sikander (5. 12. 2015). „Why is ionisation energy of bismuth lower than lead?”. chemistry.stackexchange.com. Chemistry Stack Exchange. Приступљено 5. 12. 2020. „Why is ionisation enthalpy of Bismuth less than that of Lead for it just comes after the latter in periodic table?”
- Ball, Philip (21. 4. 2017). „The group 3 dilemma”. chemistryworld.com (на језику: енглески). Burlington House, Piccadilly, London: Chemistry World. Приступљено 18. 12. 2020 — преко Royal Society of Chemistry.
- Yirka, Bob (9. 4. 2015). „Measurement of first ionization potential of lawrencium reignites debate over periodic table”. General Physics. phys.org (на језику: енглески). Phys Org. Phys.org. Приступљено 13. 12. 2020. „Lawrencium, at this time, appears to have a dumb-bell shape. These new findings create conflicting views on where the element should be placed on the table and has reignited debate on the way the table is structured in general.”
- Scerri, Eric R.; Parsons, William (март 2017). „What elements belong in group 3 of the periodic table?”. www.ionicviper.org. Ionic Viper. Приступљено 7. 12. 2020. „The question of precisely which elements should be placed in group 3 of the periodic table has been debated from time to time with apparently no resolution up to this point.”
- Sato, T. K.; Asai, M.; Borschevsky, A.; Stora, T.; Sato, N.; Kaneya, Y.; Tsukada, K.; Düllmann, Ch E.; Eberhardt, K.; Eliav, E.; Ichikawa, S.; Kaldor, U.; Kratz, J. V.; Miyashita, S.; Nagame, Y.; Ooe, K.; Osa, A.; Renisch, D.; Runke, J.; Schädel, M.; Thörle-Pospiech, P.; Toyoshima, A.; Trautmann, N. (април 2015). „Measurement of the first ionization potential of lawrencium, element 103”. Nature. 520 (7546): 209—211. Bibcode:2015Natur.520..209S. PMID 25855457. S2CID 4384213. doi:10.1038/nature14342.
- Singh, Jasvinder (1999). „Inert Gases”. Sterling Dictionary of Physics. Sterling Publishers Pvt. Ltd. стр. 122. ISBN 978-81-7359-124-2.
- „Vanadium, Niobium and Tantalum”. Chemistry of the Elements. 1997. стр. 976—1001. ISBN 978-0-7506-3365-9. doi:10.1016/B978-0-7506-3365-9.50028-6.
- Housecroft, C.E.; Sharpe, A.G. (1. 11. 1993). Inorganic Chemistry (eBook). Inorganic Chemistry (на језику: енглески). 3 (15th изд.). Switzerland: Pearson Prentice-Hall. стр. 536, 649, 743. ISBN 978-0-273-74275-3. doi:10.1021/ed070pA304.1. Архивирано из оригинала 16. 12. 2015. г. Приступљено 14. 12. 2020.
- Billo, E. J. (1985). „Modern Inorganic Chemistry (Jolly, William L.)”. Journal of Chemical Education. 62 (4): A137. Bibcode:1985JChEd..62..137B. doi:10.1021/ed062pA137.1
 .
.
Спољашње везе
- Mahan, Bruce H. (1962). „Ionization Energy”. College of Chemistry, University of California Berkeley. Приступљено 2020-09-13.
- „Monatomic Gas - an overview | ScienceDirect Topics”. www.sciencedirect.com. Приступљено 2022-01-08.
