Нуклеинске киселине — разлика између измена
м →Литература: претварање ISBN веза у шаблон |
. |
||
| Ред 1: | Ред 1: | ||
[[Датотека:RNA-comparedto-DNA thymineAndUracilCorrected.png|мини| |
[[Датотека:RNA-comparedto-DNA thymineAndUracilCorrected.png|мини|300п|Поређење две главне нуклеинске киселине: РНК (''лево'') и ДНК (''десно'')]] |
||
[[Датотека:Nucleic acid.png| |
[[Датотека:Nucleic acid.png|мини|300п|Шематски приказ структуре [[ДНК]]: жути кругови представљају [[фосфат]]е, зелени [[дезоксирибоза|дезоксирибозу]], а црвени азотне базе;пуном цртом представљена је ковалентна веза, а испрекиданом водонична]] |
||
'''Нуклеинске киселине''' су крупни и сложени органски молекули значајни за ћелију и одговорни за најзначајније процесе, као што су [[наслеђивање]], [[синтеза протеина]], у њој. |
|||
'''Нуклеинске киселине''' су крупни и сложени органски молекули значајни за ћелију и одговорни за најзначајније процесе, као што су [[наслеђивање]], [[синтеза протеина]], у њој. Постоје два типа нуклеинских киселина: [[дезоксирибонуклеинска киселина]] и [[рибонуклеинска киселина]]. ДНК је носилац наследних информација у ћелији, док РНК учествују у преношењу тих информација и њиховом превођењу у [[протеин]]е. Нуклеинске киселине су [[макромолекул]]и чију јединицу грађе представљају [[нуклеотид]]и. Њих образује један [[пентоза|пентозни]] [[шећер]] за који је везана [[Fosfat|фосфатна група]] и једна [[азотна база|азотна]], пуринска или пиримидинска база. Нуклеотиди међусобно повезују и на тај начин, захваљујући вези која се успоставља између фосфата и шећера, формирају ланац. Осим у [[вирус]]има, који садрже једну или другу нуклеинску киселину (никада обе), ДНК и РНК се налазе у свим врстама организама. Нуклеинске киселине се највише налазе у једру (lat. nucleus) па су по томе и добиле назив. Први их је изоловао [[Фридрих Мишер]] 1872. године. Нешто касније установљено је да се, осим у једру, налазе и у цитоплазми. Према данашњим подацима познато је да засебне нуклеинске киселине садрже и неке од ћелијских [[органела]], какве су нпр. [[митохондрија|митохондрије]] и [[хлоропласт]]и. Према грађи су [[полимер]]и изграђени од мономера - нуклеотида. |
|||
Постоје два типа нуклеинских киселина: |
|||
* [[дезоксирибонуклеинска киселина]] и |
|||
* [[рибонуклеинска киселина]]. |
|||
ДНК је носилац наследних информација у ћелији, док РНК учествују у преношењу тих информација и њиховом превођењу у [[протеин]]е. |
|||
Нуклеинске киселине су [[макромолекул]]и чију јединицу грађе представљају [[нуклеотид]]и. Њих образује |
|||
један [[пентоза|пентозни]] [[шећер]] за који је везана [[Fosfat|фосфатна група]] и једна [[азотна база|азотна]], пуринска или пиримидинска база. Нуклеотиди међусобно повезују и на тај начин, захваљујући вези која се успоставља између фосфата и шећера, формирају ланац. |
|||
Осим у [[вирус]]има, који садрже једну или другу нуклеинску киселину (никада обе), ДНК и РНК се налазе у свим врстама организама. |
|||
Нуклеинске киселине се највише налазе у једру (lat. nucleus) па су по томе и добиле назив. Први их је изоловао [[Фридрих Мишер]] 1872. године. Нешто касније установљено је да се, осим у једру, налазе и у цитоплазми. Према данашњим подацима познато је да засебне нуклеинске киселине садрже и неке од ћелијских [[органела]], какве су нпр. [[митохондрија|митохондрије]] и [[хлоропласт]]и. |
|||
Према грађи су [[полимер]]и изграђени од мономера - нуклеотида. |
|||
У изградњи '''нуклеотида''', који формирају '''ДНК''' учесвују: |
У изградњи '''нуклеотида''', који формирају '''ДНК''' учесвују: |
||
| Ред 27: | Ред 13: | ||
* пуринске (деривати пурина) базе аденин и гуанин, или приримидинске(деривати пиримидина) базе цитозин и [[урацил]]и |
* пуринске (деривати пурина) базе аденин и гуанин, или приримидинске(деривати пиримидина) базе цитозин и [[урацил]]и |
||
* киселински остатак фосфорне киселине. |
* киселински остатак фосфорне киселине. |
||
{{рут}} |
|||
Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and makeup the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded information is contained and conveyed via the [[nucleic acid sequence]], which provides the 'ladder-step' ordering of nucleotides within the molecules of RNA and DNA. They play an especially important role in directing protein synthesis. |
|||
Strings of nucleotides are bonded to form helical backbones—typically, one for RNA, two for DNA—and assembled into chains of base-pairs selected from the five [[nucleobase|primary, or canonical, nucleobases]], which are: [[adenine]], [[cytosine]], [[guanine]], [[thymine]], and [[uracil]]. Thymine occurs only in DNA and uracil only in RNA. Using amino acids and the process known as [[protein synthesis]],<ref>{{cite web|title=What is DNA|url=http://www.whatisdna.net|website=What is DNA|publisher=Linda Clarks|access-date=6 August 2016}}</ref> the specific sequencing in DNA of these [[base pair|nucleobase-pairs]] enables storing and transmitting [[code#Genetic code|coded]] instructions as [[gene]]s. In RNA, base-pair sequencing provides for manufacturing new proteins that determine the frames and parts and most chemical processes of all life forms. |
|||
== Историја == |
|||
[[Датотека:Friedrich Miescher.jpg|thumb|The [[Switzerland|Swiss]] [[scientist]] [[Friedrich Miescher]] discovered nucleic acids ([[DNA]]) in 1868.<ref group = "notes">He called them nuclein.</ref> Later, he raised the idea that they could be involved in [[heredity]].<ref>[[Bill Bryson]], ''[[A Short History of Nearly Everything]]'', Broadway Books, 2015.p. 500.</ref>]] |
|||
* Nuclein were discovered by [[Friedrich Miescher]] in 1869 at the [[University of Tübingen]], Germany.<ref>{{cite journal | vauthors = Dahm R | title = Discovering DNA: Friedrich Miescher and the early years of nucleic acid research | journal = Human Genetics | volume = 122 | issue = 6 | pages = 565–81 | date = January 2008 | pmid = 17901982 | doi = 10.1007/s00439-007-0433-0 | s2cid = 915930 }}</ref> |
|||
* In the early 1880s [[Albrecht Kossel]] further purified the substance and discovered its highly acidic properties. He later also identified the nucleobases. |
|||
* In '''1889''' [[Richard Altmann]] creates the term nucleic acid |
|||
* In '''1938''' [[William Astbury|Astbury]] and Bell published the first X-ray diffraction pattern of DNA.<ref>{{cite book|last1=Cox|first1=Michael|last2=Nelson|first2=David |title=Principles of Biochemistry|date=2008|publisher=Susan Winslow|page=288|url=https://books.google.com/books?id=_GUdBQAAQBAJ|isbn=9781464163074}}</ref> |
|||
* In '''1944''' the [[Avery–MacLeod–McCarty experiment]] showed that DNA is the carrier of genetic information. |
|||
* In '''1953''' [[James Watson|Watson]] and [[Francis Crick|Crick]] presented the [[Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid|structure of DNA]].<ref>{{cite web|title=DNA Structure|url=http://www.whatisdna.net/dna-structure/|website=What is DNA|publisher=Linda Clarks|access-date=6 August 2016}}</ref> |
|||
Experimental studies of nucleic acids constitute a major part of modern [[biological research|biological]] and [[medical research]], and form a foundation for [[genomics|genome]] and [[forensic science]], and the [[biotechnology]] and [[pharmaceutical industry|pharmaceutical industries]].<ref name="IHGSC">{{cite journal | vauthors = Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, etal | title = Initial sequencing and analysis of the human genome | journal = Nature | volume = 409 | issue = 6822 | pages = 860–921 | date = February 2001 | pmid = 11237011 | doi = 10.1038/35057062 | url = http://www.nature.com/nature/journal/v409/n6822/pdf/409860a0.pdf | bibcode = 2001Natur.409..860L | doi-access = free }}</ref><ref name="Venter">{{cite journal | vauthors = Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, etal | title = The sequence of the human genome | journal = Science | volume = 291 | issue = 5507 | pages = 1304–51 | date = February 2001 | pmid = 11181995 | doi = 10.1126/science.1058040 | bibcode = 2001Sci...291.1304V | doi-access = free }}</ref><ref name="Budowle">{{cite journal | vauthors = Budowle B, van Daal A | title = Extracting evidence from forensic DNA analyses: future molecular biology directions | journal = BioTechniques | volume = 46 | issue = 5 | pages = 339–40, 342–50 | date = April 2009 | pmid = 19480629 | doi = 10.2144/000113136 | doi-access = free }}</ref> |
|||
== Појава и номенклатура == |
|||
The term ''nucleic acid'' is the overall name for DNA and RNA, members of a family of [[biopolymer]]s,<ref>{{cite journal | vauthors = Elson D | journal = [[Annual Review of Biochemistry]] | volume = 34 | pages = 449–86 | year = 1965 | pmid = 14321176 | doi = 10.1146/annurev.bi.34.070165.002313 | title = Metabolism of Nucleic Acids (Macromolecular DNA and RNA) }}</ref> and is synonymous with ''[[polynucleotide]]''. Nucleic acids were named for their initial discovery within the [[Cell nucleus|nucleus]], and for the presence of phosphate groups (related to phosphoric acid).<ref>{{cite journal | vauthors = Dahm R | title = Discovering DNA: Friedrich Miescher and the early years of nucleic acid research | journal = Human Genetics | volume = 122 | issue = 6 | pages = 565–81 | date = January 2008 | pmid = 17901982 | doi = 10.1007/s00439-007-0433-0 | publisher = nih.gov | s2cid = 915930 }}</ref> Although first discovered within the [[cell nucleus|nucleus]] of [[eukaryote|eukaryotic]] cells, nucleic acids are now known to be found in all life forms including within [[bacteria]], [[archaea]], [[mitochondrion|mitochondria]], [[chloroplast]]s, and [[virus]]es (There is debate as to [[Life#Viruses|whether viruses are living or non-living]]). All living cells contain both DNA and RNA (except some cells such as mature red blood cells), while viruses contain either DNA or RNA, but usually not both.<ref name="Brock, Thomas D.; Madigan, Michael T. 2009">{{cite book | vauthors = Brock TD, Madigan MT |title=Brock biology of microorganisms |publisher=Pearson / Benjamin Cummings |year=2009 |isbn=978-0-321-53615-0 }}</ref> |
|||
The basic component of biological nucleic acids is the [[nucleotide]], each of which contains a pentose sugar ([[ribose]] or [[deoxyribose]]), a [[phosphate]] group, and a [[nucleobase]].<ref>{{cite web |url=http://www.chem.ucla.edu/harding/ec_tutorials/tutorial84.pdf |title= Knowing Nucleic Acids |author= Hardinger, Steven |author2= University of California, Los Angeles |publisher= ucla.edu |year= 2011|author2-link= University of California, Los Angeles }}</ref> |
|||
Nucleic acids are also generated within the laboratory, through the use of [[enzyme]]s<ref>Mullis, Kary B. The Polymerase Chain Reaction (Nobel Lecture). 1993. (retrieved December 1, 2010) http://nobelprize.org/nobel_prizes/chemistry/laureates/1993/mullis-lecture.html</ref> (DNA and RNA polymerases) and by [[solid-phase synthesis|solid-phase chemical synthesis]]. The chemical methods also enable the generation of altered nucleic acids that are not found in nature,<ref>{{cite journal | vauthors = Verma S, Eckstein F | title = Modified oligonucleotides: synthesis and strategy for users | journal = [[Annual Review of Biochemistry]] | volume = 67 | pages = 99–134 | year = 1998 | pmid = 9759484 | doi = 10.1146/annurev.biochem.67.1.99 | doi-access = free }}</ref> for example [[peptide nucleic acid]]s. |
|||
== Молекуларни састав и величина == |
|||
Nucleic acids are generally very large molecules. Indeed, DNA molecules are probably the largest individual molecules known. Well-studied biological nucleic acid molecules range in size from 21 nucleotides ([[small interfering RNA]]) to large chromosomes ([[chromosome 1|human chromosome 1]] is a single molecule that contains 247 million [[base pair]]s<ref>{{cite journal | vauthors = Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, etal | title = The DNA sequence and biological annotation of human chromosome 1 | journal = Nature | volume = 441 | issue = 7091 | pages = 315–21 | date = May 2006 | pmid = 16710414 | doi = 10.1038/nature04727 | bibcode = 2006Natur.441..315G | doi-access = free }}</ref>). |
|||
In most cases, naturally occurring DNA molecules are [[double helix|double-stranded]] and RNA molecules are single-stranded.<ref>{{cite journal | vauthors = Todorov TI, Morris MD | title = Comparison of RNA, single-stranded DNA and double-stranded DNA behavior during capillary electrophoresis in semidilute polymer solutions | journal = Electrophoresis | volume = 23 | issue = 7–8 | pages = 1033–44 | date = April 2002 | pmid = 11981850 | doi = 10.1002/1522-2683(200204)23:7/8<1033::AID-ELPS1033>3.0.CO;2-7 | publisher = nih.gov | others = [[National Institutes of Health]] }}</ref> There are numerous exceptions, however—some viruses have genomes made of [[Reoviridae|double-stranded RNA]] and other viruses have [[M13 bacteriophage|single-stranded DNA]] genomes,<ref>{{cite web |url=http://pathmicro.med.sc.edu/mhunt/rna-ho.htm |title= RN Virus Replication Strategies |author= Margaret Hunt |author2= University of South Carolina |publisher= sc.edu |year= 2010|author2-link= University of South Carolina }}</ref> and, in some circumstances, nucleic acid structures with [[triple-stranded DNA|three]] or [[G-quadruplex|four]] strands can form.<ref name="pmid10454599">{{cite journal | vauthors = McGlynn P, Lloyd RG | title = RecG helicase activity at three- and four-strand DNA structures | journal = Nucleic Acids Research | volume = 27 | issue = 15 | pages = 3049–56 | date = August 1999 | pmid = 10454599 | pmc = 148529 | doi = 10.1093/nar/27.15.3049}}</ref> |
|||
Nucleic acids are linear [[polymer]]s (chains) of nucleotides. Each nucleotide consists of three components: a [[purine]] or [[pyrimidine]] [[nucleobase]] (sometimes termed ''nitrogenous base'' or simply ''base''), a [[pentose]] [[sugar]], and a [[phosphate]] group which makes the molecule acidic. The substructure consisting of a nucleobase plus sugar is termed a [[nucleoside]]. Nucleic acid types differ in the structure of the sugar in their nucleotides–DNA contains 2'-[[deoxyribose]] while RNA contains [[ribose]] (where the only difference is the presence of a [[hydroxyl group]]). Also, the nucleobases found in the two nucleic acid types are different: [[adenine]], [[cytosine]], and [[guanine]] are found in both RNA and DNA, while [[thymine]] occurs in DNA and [[uracil]] occurs in RNA. |
|||
The sugars and phosphates in nucleic acids are connected to each other in an alternating chain (sugar-phosphate backbone) through [[phosphodiester]] linkages.<ref name="Stryer">{{cite book |author1=Stryer, Lubert |author2=Berg, Jeremy Mark |author3=Tymoczko, John L. |title=Biochemistry |publisher=W.H. Freeman |location=San Francisco |year=2007 |isbn=978-0-7167-6766-4 |url-access=registration |url=https://archive.org/details/biochemistry0006berg }}</ref> In [[nucleic acid nomenclature|conventional nomenclature]], the carbons to which the phosphate groups attach are the 3'-end and the 5'-end carbons of the sugar. This gives nucleic acids [[directionality (molecular biology)|directionality]], and the ends of nucleic acid molecules are referred to as 5'-end and 3'-end. The nucleobases are joined to the sugars via an N-glycosidic linkage involving a nucleobase ring nitrogen (N-1 for pyrimidines and N-9 for purines) and the 1' carbon of the pentose sugar ring. |
|||
Non-standard nucleosides are also found in both RNA and DNA and usually arise from modification of the standard nucleosides within the DNA molecule or the primary (initial) RNA transcript. [[Transfer RNA]] (tRNA) molecules contain a particularly large number of modified nucleosides.<ref>{{cite journal | vauthors = Rich A, RajBhandary UL | title = Transfer RNA: molecular structure, sequence, and properties | journal = [[Annual Review of Biochemistry]] | volume = 45 | pages = 805–60 | year = 1976 | pmid = 60910 | doi = 10.1146/annurev.bi.45.070176.004105 }}</ref> |
|||
== Напомене == |
|||
{{reflist|group=notes}} |
|||
== Референце == |
|||
{{reflist|}} |
|||
== Литература == |
== Литература == |
||
{{refbegin|2}} |
{{refbegin|2}} |
||
* Wolfram Saenger, ''Principles of Nucleic Acid Structure'', 1984, Springer-Verlag New York Inc. |
* Wolfram Saenger, ''Principles of Nucleic Acid Structure'', 1984, Springer-Verlag New York Inc. |
||
* Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter ''Molecular Biology of the Cell''. 2007. {{ISBN|978-0-8153-4105-5}}. Fourth edition is available online through the NCBI Bookshelf: [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4 link] |
* Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter ''Molecular Biology of the Cell''. 2007. {{ISBN|978-0-8153-4105-5}}. Fourth edition is available online through the NCBI Bookshelf: [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4 link] |
||
* Jeremy M Berg, John L Tymoczko, and Lubert Stryer, ''Biochemistry'' 5th edition, 2002, W H Freeman. Available online through the NCBI Bookshelf: [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer link] |
* Jeremy M Berg, John L Tymoczko, and Lubert Stryer, ''Biochemistry'' 5th edition, 2002, W H Freeman. Available online through the NCBI Bookshelf: [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer link] |
||
* {{Cite book |ref= harv| |
* {{Cite book |ref= harv |editor= Astrid Sigel |editor2= Helmut Sigel |editor3= Roland K. O. Sigel |title=Interplay between Metal Ions and Nucleic Acids|series=Metal Ions in Life Sciences|volume=10|year=2012|publisher=Springer|doi=10.1007/978-94-007-2172-2|isbn=978-94-007-2171-5}} |
||
* {{cite book | last1 = Palou-Mir | first1 = Joana | last2 = Barceló-Oliver | first2 = Miquel | last3 = Sigel | first3 = Roland K.O. | chapter = Chapter 12. The Role of Lead(II) in Nucleic Acids | pages = 403–434 | publisher = de Gruyter | date = 2017 | series = Metal Ions in Life Sciences | volume = 17 | title = Lead: Its Effects on Environment and Health | editor1-last = Astrid | editor1-first = S. | editor2-last = Helmut | editor2-first = S. | editor3-last = Sigel | editor3-first = R. K. O. | doi = 10.1515/9783110434330-012 | pmid = 28731305 }} |
|||
{{refend}} |
|||
== Спољашње везе == |
== Спољашње везе == |
||
| Ред 42: | Ред 67: | ||
* [http://nar.oxfordjournals.org/ -{Nucleic Acids Research (Journal)}-] |
* [http://nar.oxfordjournals.org/ -{Nucleic Acids Research (Journal)}-] |
||
* [http://www.atdbio.com/nucleic-acids-book -{Nucleic Acids Book (free online book on the chemistry and biology of nucleic acids)}-] |
* [http://www.atdbio.com/nucleic-acids-book -{Nucleic Acids Book (free online book on the chemistry and biology of nucleic acids)}-] |
||
{{-}} |
|||
{{Нуклеинске киселине}} |
|||
{{Биомолекуларне структуре}} |
{{Биомолекуларне структуре}} |
||
{{Authority control}} |
|||
{{Portal bar|Биологина}} |
|||
[[Категорија:Молекуларна биологија]] |
[[Категорија:Молекуларна биологија]] |
||
Верзија на датум 14. јун 2021. у 21:53
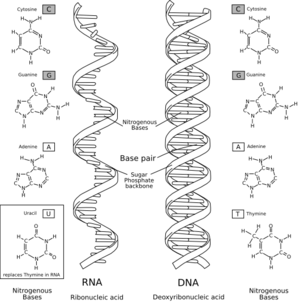

Нуклеинске киселине су крупни и сложени органски молекули значајни за ћелију и одговорни за најзначајније процесе, као што су наслеђивање, синтеза протеина, у њој. Постоје два типа нуклеинских киселина: дезоксирибонуклеинска киселина и рибонуклеинска киселина. ДНК је носилац наследних информација у ћелији, док РНК учествују у преношењу тих информација и њиховом превођењу у протеине. Нуклеинске киселине су макромолекули чију јединицу грађе представљају нуклеотиди. Њих образује један пентозни шећер за који је везана фосфатна група и једна азотна, пуринска или пиримидинска база. Нуклеотиди међусобно повезују и на тај начин, захваљујући вези која се успоставља између фосфата и шећера, формирају ланац. Осим у вирусима, који садрже једну или другу нуклеинску киселину (никада обе), ДНК и РНК се налазе у свим врстама организама. Нуклеинске киселине се највише налазе у једру (lat. nucleus) па су по томе и добиле назив. Први их је изоловао Фридрих Мишер 1872. године. Нешто касније установљено је да се, осим у једру, налазе и у цитоплазми. Према данашњим подацима познато је да засебне нуклеинске киселине садрже и неке од ћелијских органела, какве су нпр. митохондрије и хлоропласти. Према грађи су полимери изграђени од мономера - нуклеотида.
У изградњи нуклеотида, који формирају ДНК учесвују:
- пентозни шећер дезоксирибоза,
- пуринске (деривати пурина) базе аденин и гуанин, или приримидинске(деривати пиримидин) базе цитозин и тимин
- киселински остатак фосфорне киселине.
У изградњи нуклеотида РНК учесвују:
- пентозни шећер рибоза,
- пуринске (деривати пурина) базе аденин и гуанин, или приримидинске(деривати пиримидина) базе цитозин и урацили
- киселински остатак фосфорне киселине.
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and makeup the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded information is contained and conveyed via the nucleic acid sequence, which provides the 'ladder-step' ordering of nucleotides within the molecules of RNA and DNA. They play an especially important role in directing protein synthesis.
Strings of nucleotides are bonded to form helical backbones—typically, one for RNA, two for DNA—and assembled into chains of base-pairs selected from the five primary, or canonical, nucleobases, which are: adenine, cytosine, guanine, thymine, and uracil. Thymine occurs only in DNA and uracil only in RNA. Using amino acids and the process known as protein synthesis,[1] the specific sequencing in DNA of these nucleobase-pairs enables storing and transmitting coded instructions as genes. In RNA, base-pair sequencing provides for manufacturing new proteins that determine the frames and parts and most chemical processes of all life forms.
Историја

- Nuclein were discovered by Friedrich Miescher in 1869 at the University of Tübingen, Germany.[3]
- In the early 1880s Albrecht Kossel further purified the substance and discovered its highly acidic properties. He later also identified the nucleobases.
- In 1889 Richard Altmann creates the term nucleic acid
- In 1938 Astbury and Bell published the first X-ray diffraction pattern of DNA.[4]
- In 1944 the Avery–MacLeod–McCarty experiment showed that DNA is the carrier of genetic information.
- In 1953 Watson and Crick presented the structure of DNA.[5]
Experimental studies of nucleic acids constitute a major part of modern biological and medical research, and form a foundation for genome and forensic science, and the biotechnology and pharmaceutical industries.[6][7][8]
Појава и номенклатура
The term nucleic acid is the overall name for DNA and RNA, members of a family of biopolymers,[9] and is synonymous with polynucleotide. Nucleic acids were named for their initial discovery within the nucleus, and for the presence of phosphate groups (related to phosphoric acid).[10] Although first discovered within the nucleus of eukaryotic cells, nucleic acids are now known to be found in all life forms including within bacteria, archaea, mitochondria, chloroplasts, and viruses (There is debate as to whether viruses are living or non-living). All living cells contain both DNA and RNA (except some cells such as mature red blood cells), while viruses contain either DNA or RNA, but usually not both.[11] The basic component of biological nucleic acids is the nucleotide, each of which contains a pentose sugar (ribose or deoxyribose), a phosphate group, and a nucleobase.[12] Nucleic acids are also generated within the laboratory, through the use of enzymes[13] (DNA and RNA polymerases) and by solid-phase chemical synthesis. The chemical methods also enable the generation of altered nucleic acids that are not found in nature,[14] for example peptide nucleic acids.
Молекуларни састав и величина
Nucleic acids are generally very large molecules. Indeed, DNA molecules are probably the largest individual molecules known. Well-studied biological nucleic acid molecules range in size from 21 nucleotides (small interfering RNA) to large chromosomes (human chromosome 1 is a single molecule that contains 247 million base pairs[15]).
In most cases, naturally occurring DNA molecules are double-stranded and RNA molecules are single-stranded.[16] There are numerous exceptions, however—some viruses have genomes made of double-stranded RNA and other viruses have single-stranded DNA genomes,[17] and, in some circumstances, nucleic acid structures with three or four strands can form.[18]
Nucleic acids are linear polymers (chains) of nucleotides. Each nucleotide consists of three components: a purine or pyrimidine nucleobase (sometimes termed nitrogenous base or simply base), a pentose sugar, and a phosphate group which makes the molecule acidic. The substructure consisting of a nucleobase plus sugar is termed a nucleoside. Nucleic acid types differ in the structure of the sugar in their nucleotides–DNA contains 2'-deoxyribose while RNA contains ribose (where the only difference is the presence of a hydroxyl group). Also, the nucleobases found in the two nucleic acid types are different: adenine, cytosine, and guanine are found in both RNA and DNA, while thymine occurs in DNA and uracil occurs in RNA.
The sugars and phosphates in nucleic acids are connected to each other in an alternating chain (sugar-phosphate backbone) through phosphodiester linkages.[19] In conventional nomenclature, the carbons to which the phosphate groups attach are the 3'-end and the 5'-end carbons of the sugar. This gives nucleic acids directionality, and the ends of nucleic acid molecules are referred to as 5'-end and 3'-end. The nucleobases are joined to the sugars via an N-glycosidic linkage involving a nucleobase ring nitrogen (N-1 for pyrimidines and N-9 for purines) and the 1' carbon of the pentose sugar ring.
Non-standard nucleosides are also found in both RNA and DNA and usually arise from modification of the standard nucleosides within the DNA molecule or the primary (initial) RNA transcript. Transfer RNA (tRNA) molecules contain a particularly large number of modified nucleosides.[20]
Напомене
- ^ He called them nuclein.
Референце
- ^ „What is DNA”. What is DNA. Linda Clarks. Приступљено 6. 8. 2016.
- ^ Bill Bryson, A Short History of Nearly Everything, Broadway Books, 2015.p. 500.
- ^ Dahm R (јануар 2008). „Discovering DNA: Friedrich Miescher and the early years of nucleic acid research”. Human Genetics. 122 (6): 565—81. PMID 17901982. S2CID 915930. doi:10.1007/s00439-007-0433-0.
- ^ Cox, Michael; Nelson, David (2008). Principles of Biochemistry. Susan Winslow. стр. 288. ISBN 9781464163074.
- ^ „DNA Structure”. What is DNA. Linda Clarks. Приступљено 6. 8. 2016.
- ^ Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. (фебруар 2001). „Initial sequencing and analysis of the human genome” (PDF). Nature. 409 (6822): 860—921. Bibcode:2001Natur.409..860L. PMID 11237011. doi:10.1038/35057062
 .
.
- ^ Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (фебруар 2001). „The sequence of the human genome”. Science. 291 (5507): 1304—51. Bibcode:2001Sci...291.1304V. PMID 11181995. doi:10.1126/science.1058040
 .
.
- ^ Budowle B, van Daal A (април 2009). „Extracting evidence from forensic DNA analyses: future molecular biology directions”. BioTechniques. 46 (5): 339—40, 342—50. PMID 19480629. doi:10.2144/000113136
 .
.
- ^ Elson D (1965). „Metabolism of Nucleic Acids (Macromolecular DNA and RNA)”. Annual Review of Biochemistry. 34: 449—86. PMID 14321176. doi:10.1146/annurev.bi.34.070165.002313.
- ^ Dahm R (јануар 2008). „Discovering DNA: Friedrich Miescher and the early years of nucleic acid research”. Human Genetics. nih.gov. 122 (6): 565—81. PMID 17901982. S2CID 915930. doi:10.1007/s00439-007-0433-0.
- ^ Brock TD, Madigan MT (2009). Brock biology of microorganisms. Pearson / Benjamin Cummings. ISBN 978-0-321-53615-0.
- ^ Hardinger, Steven; University of California, Los Angeles (2011). „Knowing Nucleic Acids” (PDF). ucla.edu.
- ^ Mullis, Kary B. The Polymerase Chain Reaction (Nobel Lecture). 1993. (retrieved December 1, 2010) http://nobelprize.org/nobel_prizes/chemistry/laureates/1993/mullis-lecture.html
- ^ Verma S, Eckstein F (1998). „Modified oligonucleotides: synthesis and strategy for users”. Annual Review of Biochemistry. 67: 99—134. PMID 9759484. doi:10.1146/annurev.biochem.67.1.99
 .
.
- ^ Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, et al. (мај 2006). „The DNA sequence and biological annotation of human chromosome 1”. Nature. 441 (7091): 315—21. Bibcode:2006Natur.441..315G. PMID 16710414. doi:10.1038/nature04727
 .
.
- ^ Todorov TI, Morris MD (април 2002). National Institutes of Health. „Comparison of RNA, single-stranded DNA and double-stranded DNA behavior during capillary electrophoresis in semidilute polymer solutions”. Electrophoresis. nih.gov. 23 (7–8): 1033—44. PMID 11981850. doi:10.1002/1522-2683(200204)23:7/8<1033::AID-ELPS1033>3.0.CO;2-7.
- ^ Margaret Hunt; University of South Carolina (2010). „RN Virus Replication Strategies”. sc.edu.
- ^ McGlynn P, Lloyd RG (август 1999). „RecG helicase activity at three- and four-strand DNA structures”. Nucleic Acids Research. 27 (15): 3049—56. PMC 148529
 . PMID 10454599. doi:10.1093/nar/27.15.3049.
. PMID 10454599. doi:10.1093/nar/27.15.3049.
- ^ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2007). Biochemistry
 . San Francisco: W.H. Freeman. ISBN 978-0-7167-6766-4.
. San Francisco: W.H. Freeman. ISBN 978-0-7167-6766-4.
- ^ Rich A, RajBhandary UL (1976). „Transfer RNA: molecular structure, sequence, and properties”. Annual Review of Biochemistry. 45: 805—60. PMID 60910. doi:10.1146/annurev.bi.45.070176.004105.
Литература
- Wolfram Saenger, Principles of Nucleic Acid Structure, 1984, Springer-Verlag New York Inc.
- Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter Molecular Biology of the Cell. 2007. ISBN 978-0-8153-4105-5. Fourth edition is available online through the NCBI Bookshelf: link
- Jeremy M Berg, John L Tymoczko, and Lubert Stryer, Biochemistry 5th edition, 2002, W H Freeman. Available online through the NCBI Bookshelf: link
- Astrid Sigel; Helmut Sigel; Roland K. O. Sigel, ур. (2012). Interplay between Metal Ions and Nucleic Acids. Metal Ions in Life Sciences. 10. Springer. ISBN 978-94-007-2171-5. doi:10.1007/978-94-007-2172-2.
- Palou-Mir, Joana; Barceló-Oliver, Miquel; Sigel, Roland K.O. (2017). „Chapter 12. The Role of Lead(II) in Nucleic Acids”. Ур.: Astrid, S.; Helmut, S.; Sigel, R. K. O. Lead: Its Effects on Environment and Health. Metal Ions in Life Sciences. 17. de Gruyter. стр. 403—434. PMID 28731305. doi:10.1515/9783110434330-012.
Спољашње везе
- Бионет школа
- Interview with Aaron Klug, Nobel Laureate for structural elucidation of biologically important nucleic-acid protein complexes provided by the Vega Science Trust.
- Nucleic Acids Research (Journal)
- Nucleic Acids Book (free online book on the chemistry and biology of nucleic acids)
