Бекерел — разлика између измена
#1Lib1Ref |
. |
||
| Ред 1: | Ред 1: | ||
{{Short description|СИ изведена јединица радиоактивности}}{{рут}} |
|||
{{Infobox Unit |
|||
| name = Бекерел |
|||
| standard = [[Изведене јединице СИ система]] |
|||
| quantity = [[Specific activity|Активност]] |
|||
| symbol = Bq |
|||
| namedafter = [[Henri Becquerel|Анри Бекерел]] |
|||
| units1 = [[Rutherford (unit)|радерфорд]] |
|||
| inunits1 = {{val|e=-6|u=Rd}} |
|||
| units2 = [[Curie (unit)|кири]] |
|||
| inunits2 = {{val|2.703|e=-11|u=Ci}} ≅ {{val|27|u=pCi}} |
|||
| units3 = [[SI base unit|СИ основне јединице]] |
|||
| inunits3 = [[second|s]]<sup>−1</sup> |
|||
}} |
|||
{{остале употребе}} |
{{остале употребе}} |
||
'''Бекерел''' ({{јез-ен|becquerel}}; симбол: '''Bq''') [[СИ изведене јединице|изведена је јединица СИ система]] која се дефинише као активност количине радиоактивног материјала где се једно [[атомско језгро|језгро]] распада у [[секунд]]и.<ref>{{cite web |title=Radioactivity : Radioactive Activity Doses |url=https://www.radioactivity.eu.com/site/pages/Activity_Doses.htm |website=www.radioactivity.eu.com |access-date=20 February 2020}}</ref> Бекерел је, стога, еквивалентан [[секунд|s]]<sup>-1</sup>. Старија јединица радиоактивности је била [[кири]] (Ci), дефинисана као 37×10<sup>9</sup> бекерела или 37 GBq.<ref>{{cite web |title=Radiation Protection Guidance For Hospital Staff – Stanford Environmental Health & Safety |url=https://ehs.stanford.edu/manual/radiation-protection-guidance-hospital-staff/what-are-units-radiation-activity |website=ehs.stanford.edu |access-date=20 February 2020}}</ref> |
'''Бекерел''' ({{јез-ен|becquerel}}; симбол: '''Bq''') [[СИ изведене јединице|изведена је јединица СИ система]] која се дефинише као активност количине радиоактивног материјала где се једно [[атомско језгро|језгро]] распада у [[секунд]]и.<ref>{{cite web |title=Radioactivity : Radioactive Activity Doses |url=https://www.radioactivity.eu.com/site/pages/Activity_Doses.htm |website=www.radioactivity.eu.com |access-date=20 February 2020}}</ref> Бекерел је, стога, еквивалентан [[секунд|s]]<sup>-1</sup>. Старија јединица радиоактивности је била [[кири]] (Ci), дефинисана као 37×10<sup>9</sup> бекерела или 37 GBq.<ref>{{cite web |title=Radiation Protection Guidance For Hospital Staff – Stanford Environmental Health & Safety |url=https://ehs.stanford.edu/manual/radiation-protection-guidance-hospital-staff/what-are-units-radiation-activity |website=ehs.stanford.edu |access-date=20 February 2020}}</ref> |
||
Назван је по [[Анри Бекерел|Анрију Бекерелу]], који је делио [[Нобелова награда|Нобелову награду]] с [[Марија Кири|Маријом Кири]] због њиховог рада у откривању радиоактивности. |
Назван је по [[Анри Бекерел|Анрију Бекерелу]], који је делио [[Нобелова награда|Нобелову награду]] с [[Марија Кири|Маријом Кири]] због њиховог рада у откривању радиоактивности.<ref name="BIPMBecquerel">{{cite web|url=http://www.bipm.org/en/si/history-si/radioactivity/becquerel.html|title=BIPM - Becquerel|publisher=[[BIPM]]|access-date=2012-10-24}}</ref> |
||
За фиксирану масу радиоактивног метала, број бекерела се мења временом. Под неким околностима, количине радиоактивног материјала су дате после неког времена за подешавање. На пример, може да се узме десетодневна подешена цифра, тј. количина радиоактивности која ће и даље бити присутна после десет дана. Ово склања нагласак са кратковековних изотопа. |
За фиксирану масу радиоактивног метала, број бекерела се мења временом. Под неким околностима, количине радиоактивног материјала су дате после неког времена за подешавање. На пример, може да се узме десетодневна подешена цифра, тј. количина радиоактивности која ће и даље бити присутна после десет дана. Ово склања нагласак са кратковековних изотопа. |
||
Намеравало се да се бекерел користи у [[Међународни систем јединица|СИ-ју]], уместо реципрочне секунде, као јединица мерења активности. Ово је посебно уведено због опасности по људско здравље које могу да проистекну услед грешака везане за реципрочну секунду. Користећи бекерел, активнији извор има већи број (па је и опасније). Користећи 1/s или s као секунду може да доведе до конфузије. |
Намеравало се да се бекерел користи у [[Међународни систем јединица|СИ-ју]], уместо реципрочне секунде, као јединица мерења активности. Ово је посебно уведено због опасности по људско здравље које могу да проистекну услед грешака везане за реципрочну секунду. Користећи бекерел, активнији извор има већи број (па је и опасније). Користећи 1/s или s као секунду може да доведе до конфузије. |
||
== Дефиниција == |
|||
1 Bq = 1 s<sup>−1</sup> |
|||
A special name was introduced for the [[Inverse second|reciprocal second]] (s<sup>−1</sup>) to represent radioactivity to avoid potentially dangerous mistakes with prefixes. For example, 1 µs<sup>−1</sup> would mean 10<sup>6</sup> disintegrations per second: 1·(10<sup>−6</sup> s)<sup>−1</sup> = 10<sup>6</sup> s<sup>−1</sup>,<ref name="Allisy">{{Citation|last=Allisy|first= A.|title=From the curie to the becquerel|journal=Metrologia|volume=32|issue=6|year=1995|pages=467–479|doi=10.1088/0026-1394/31/6/006|bibcode = 1995Metro..31..467A}}</ref> whereas 1 µBq would mean 1 disintegration per 1 million seconds. Other names considered were [[hertz]] (Hz), a special name already in use for the reciprocal second, and [[Joseph Fourier|Fourier]] (Fr).<ref name="Allisy" /> The hertz is now only used for periodic phenomena.<ref name="BIPMtable3">{{cite web|url=http://www.bipm.org/en/publications/si-brochure/section2-2.html#section2-2-2|title=BIPM - Table 3|publisher=[[BIPM]]|quote=(d) The hertz is used only for periodic phenomena, and the becquerel is used only for stochastic processes in activity referred to a radionuclide.|access-date=2015-07-19}}</ref> Whereas 1 Hz is 1 [[cycle per second]], 1 Bq is 1 [[aperiodic frequency|aperiodic]] radioactivity event per second. |
|||
The [[gray (unit)|gray]] (Gy) and the becquerel (Bq) were introduced in 1975.<ref name="pmid_1251122">{{Citation|last=Harder|first=D|year=1976|title=[The new radiologic units of measurement gray and becquerel (author's translation from the German original)]|journal=Röntgen-Blätter|volume=29|issue=1 |pages=49–52 |pmid=1251122 |postscript=.}}</ref> Between 1953 and 1975, absorbed dose was often measured in [[rad (unit)|rads]]. Decay activity was measured in [[Curie (unit)|curie]]s before 1946 and often in [[rutherford (unit)|rutherfords]] between 1946<ref name="pmid_17836457">{{Citation|last=Lind|first=SC|year=1946|title=New units for the measurement of radioactivity|journal=Science|volume=103|issue=2687|pages=761–762|pmid=17836457|doi=10.1126/science.103.2687.761-a|postscript=.|bibcode=1946Sci...103..761L |s2cid=5343688 }}</ref> and 1975. |
|||
== Велика слова и префикси јединица == |
|||
As with every International System of Units (SI) unit named for a person, the first letter of its symbol is uppercase (Bq). However, when an SI unit is spelled out in English, it should always begin with a lowercase letter (becquerel)—except in a situation where any word in that position would be capitalized, such as at the beginning of a sentence or in material using [[title case]].<ref> |
|||
{{cite web|title=SI Brochure: The International System of Units (SI)|url=http://www.bipm.org/en/publications/si-brochure/section5-2.html|edition=8|date=2014|work=SI Brochure|publisher=[[BIPM]]}}</ref> |
|||
Like any SI unit, Bq can be [[SI prefix|prefixed]]; commonly used multiples are kBq (kilobecquerel, 10<sup>3</sup> Bq), MBq (megabecquerel, 10<sup>6</sup> Bq, equivalent to 1 [[rutherford (unit)|rutherford]]), GBq (gigabecquerel, 10<sup>9</sup> Bq), TBq (terabecquerel, 10<sup>12</sup> Bq), and PBq (petabecquerel, 10<sup>15</sup> Bq). Large prefixes are common for practical uses of the unit. |
|||
== Прорачун радиоактивности == |
|||
For a given mass <math>m</math> (in grams) of an isotope with [[atomic mass]] <math>m_\text{a}</math> (in g/mol) and a [[half-life]] of <math>t_{1/2}</math> (in s), the radioactivity can be calculated using: |
|||
<math>A_\text{Bq} = \frac{m} {m_\text{a}} N_\text{A} \frac{\ln 2} {t_{1/2}}</math> |
|||
With <math>N_\text{A}</math> = {{val|6.02214076|e=23|u=mol-1}}, the [[Avogadro constant]]. |
|||
Since <math>m/m_\text{a}</math> is the number of moles (<math>n</math>), the amount of radioactivity <math>A</math> can be calculated by: |
|||
<math>A_\text{Bq} = nN_\text{A} \frac{\ln 2} {t_{1/2}}</math> |
|||
For instance, on average each gram of [[potassium]] contains 117 micrograms of [[Potassium-40|<sup>40</sup>K]] (all other naturally occurring isotopes are stable) that has a <math>t_{1/2}</math> of {{val|1.277|e=9|u=years}} = {{val|4.030|e=16|u=s}},<ref>{{cite web|url=http://nucleardata.nuclear.lu.se/toi/nuclide.asp?iZA=190040 |title=Table of Isotopes decay data |publisher=[[Lund University]] |date=1990-06-01 |access-date=2014-01-12}}</ref> and has an atomic mass of 39.964 g/mol,<ref>{{cite web|url=http://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl?ele=&ascii=html&isotype=some |title=Atomic Weights and Isotopic Compositions for All Elements |publisher=[[NIST]] |access-date=2014-01-12}}</ref> so the amount of radioactivity associated with a gram of potassium is 30 Bq. |
|||
== Примери == |
|||
For practical applications, 1 Bq is a small unit. For example, the roughly 0.0169 g of [[potassium-40]] present in a typical human body produces approximately 4,400 disintegrations per second or 4.4 kBq of activity.<ref>[http://www.fas.harvard.edu/~scdiroff/lds/QuantumRelativity/RadioactiveHumanBody/RadioactiveHumanBody.html ''Radioactive human body'' — Harvard University Natural Science Lecture Demonstrations] - Accessed October 2013</ref> |
|||
The global inventory of [[carbon-14]] is estimated to be {{val|8.5|e=18|u=Bq}} (8.5 [[Exa-|E]]Bq, 8.5 exabecquerel).<ref>G.R. Choppin, [[Jan-Olov Liljenzin|J.O.Liljenzin]], J. Rydberg, "Radiochemistry and Nuclear Chemistry", 3rd edition, Butterworth-Heinemann, 2002. {{ISBN|978-0-7506-7463-8}}.</ref> |
|||
The [[Atomic bombings of Hiroshima and Nagasaki|nuclear explosion]] in [[Hiroshima]] (an explosion of {{convert|16|kt(TNT)|lk=in|abbr=on|disp=or}}) is estimated to have injected {{val|8|e=24|u=Bq}} (8 [[Yotta-|Y]]Bq, 8 yottabecquerel) of radioactive fission products into the atmosphere.<ref>{{Cite book|last=Harrison|url=https://www.worldcat.org/oclc/869096285|title=Pollution : Causes, Effects and Control.|date=2013|publisher=Royal Society of Chemistry|isbn=978-1-68015-810-6|location=Cambridge|oclc=869096285}}</ref> |
|||
These examples are useful for comparing the amount of activity of these radioactive materials but should not be confused with the amount of exposure to ionizing radiation that these materials represent. The level of exposure and thus the [[absorbed dose]] received are what should be considered when assessing the effects of ionizing radiation on humans. |
|||
== Релација са киријом == |
|||
The becquerel succeeded the [[Curie (unit)|curie]] (Ci),<ref>It was adopted by the BIPM in 1975, see [http://www.bipm.org/en/CGPM/db/15/8/ resolution 8 of the 15th CGPM meeting]</ref> an older, non-SI unit of radioactivity based on the activity of 1 gram of [[radium-226]]. The curie is defined as {{val|3.7|e=10|u=s-1}}, or 37 GBq.<ref name="Allisy" /><ref>[https://www.bipm.org/en/CGPM/db/12/7/ Resolution 7 of the 12th CGPM] {{Webarchive|url=https://web.archive.org/web/20210219084448/https://www.bipm.org/en/CGPM/db/12/7 |date=2021-02-19 }} (1964)</ref> |
|||
Conversion factors: |
|||
: 1 Ci = {{val|3.7|e=10|u=Bq}} = 37 GBq |
|||
: 1 μCi = 37,000 Bq = 37 kBq |
|||
: 1 Bq = {{val|2.7|e=-11|u=Ci}} = {{val|2.7|e=-5|u=µCi}} |
|||
: 1 MBq = 0.027 mCi |
|||
== Однос према другим величинама у вези са зрачењем == |
|||
[[File:Radioactivity and radiation.png|thumb|upright=2|Graphic showing relationships between radioactivity and detected ionizing radiation]] |
|||
The following table shows radiation quantities in SI and non-SI units. [[Sievert#Calculating protection dose quantities|''W''<sub>''R''</sub>]] (formerly 'Q' factor) is a factor that scales the biological effect for different types of radiation, relative to x-rays. (e.g. 1 for beta radiation, 20 for alpha radiation, and a complicated function of energy for neutrons) |
|||
In general conversion between rates of emission, the density of radiation, the fraction absorbed, and the biological effects, requires knowledge of the geometry between source and target, the energy and the type of the radiation emitted, among other factors.<ref>http://hps.org/publicinformation/ate/faqs/gammaandexposure.html</ref> |
|||
| ⚫ | |||
| ⚫ | |||
== Литература == |
== Литература == |
||
{{refbegin| |
{{refbegin|30em}} |
||
* {{GreenBook3rd}} |
* {{GreenBook3rd}} |
||
* {{GreenBook2nd}} |
* {{GreenBook2nd}} |
||
* {{cite book|title = IAEA Safety Glossary: Terminology Used in Nuclear Safety and Radiation Protection |date = 2007|author = International Atomic Energy Agency|isbn = 9789201007070}} |
|||
* {{cite book |author=United Nations Scientific Committee on the Effects of Atomic Radiation|title=Sources and effects of ionizing radiation |date=2008 |publication-date=2010 |publisher=United Nations |location=New York |isbn=978-92-1-142274-0 |url=http://www.unscear.org/unscear/en/publications/2008_1.html |access-date=9 November 2012|page=4}} |
|||
* {{cite book|title=Ionizing radiation exposure of the population of the United States|year=2009|publisher=National Council on Radiation Protection and Measurements|location=Bethesda, Md.|isbn=978-0-929600-98-7|id=NCRP No. 160|url=http://www.ncrppublications.org/Reports/160|access-date=9 November 2012|archive-date=2 February 2014|archive-url=https://web.archive.org/web/20140202092721/http://www.ncrppublications.org/Reports/160|url-status=dead}} |
|||
* Ministry of Education, Culture, Sports, Science, and Technology of Japan [http://www.kankyo-hoshano.go.jp/04/04-1.html "Radiation in environment"] {{Webarchive|url=https://web.archive.org/web/20110322231148/http://www.kankyo-hoshano.go.jp/04/04-1.html |date=22 March 2011 }} |
|||
* {{cite web|url=http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Naturally-Occurring-Radioactive-Materials-NORM/|title= Naturally-Occurring Radioactive Materials (NORM)|date = March 2019|website =World Nuclear Association}} |
|||
* {{cite web |url = http://www-ns.iaea.org/tech-areas/rw-ppss/exposure-to-natural-radiation.asp?s=3 |publisher = IAEA |website = Nuclear Safety & Security |title = Exposure to radiation from natural sources |archive-url = https://web.archive.org/web/20160209112421/http://www-ns.iaea.org/tech-areas/rw-ppss/exposure-to-natural-radiation.asp?s=3 |archive-date = 9 February 2016 |access-date = 4 January 2016 |url-status = live }} |
|||
* {{cite book|author=United Nations Scientific Committee on the Effects of Atomic Radiation|title=Effects of Ionizing Radiation |date=2006 |publication-date=2008 |publisher=United Nations|location=New York|isbn=978-92-1-142263-4|volume=II|chapter=Annex E: Sources-to-effects assessment for radon in homes and workplaces|chapter-url=http://www.unscear.org/docs/reports/2006/09-81160_Report_Annex_E_2006_Web.pdf|access-date=2 December 2012}} |
|||
* {{cite web| url = http://www.cancer.gov/cancertopics/factsheet/Risk/radon| title = Radon and Cancer: Questions and Answers – National Cancer Institute (USA)| date = 6 December 2011}} |
|||
* {{cite journal |last=Fornalski |first=K. W. |author2=Adams, R. |author3=Allison, W. |author4=Corrice, L. E. |author5=Cuttler, J. M. |author6=Davey, Ch. |author7=Dobrzyński, L. |author8=Esposito, V. J. |author9=Feinendegen, L. E. |author10=Gomez, L. S. |author11=Lewis, P. |author12=Mahn, J. |author13=Miller, M. L. |author14=Pennington, Ch. W. |author15=Sacks, B. |author16=Sutou, S. |author17=Welsh, J. S. |pmid=26223888 |title=The assumption of radon-induced cancer risk |year=2015 |journal=Cancer Causes & Control |doi=10.1007/s10552-015-0638-9 |issue=26 |volume=10 |pages=1517–18|s2cid=15952263 }} |
|||
* {{cite conference |url=http://wpb-radon.com/Radon_research_papers/1995%20Nashville,%20TN/1995_14_Indoor%20Radon%20Concentration%20Data--Geographic%20and%20Geologic%20Distribution,%20Captial%20District,%20NY.pdf |title=Indoor Radon Concentration Data: Its Geographic and Geologic Distribution, an Example from the Capital District, NY |first1=John J. |last1=Thomas |first2=Barbara R. |last2=Thomas |first3=Helen M. |last3=Overeynder |date= 27–30 September 1995 |conference=International Radon Symposium |conference-url=http://internationalradonsymposium.org/ |publisher=American Association of Radon Scientists and Technologists |location=Nashville, TN |access-date=2012-11-28}} |
|||
* {{cite book|last1=Upfal |first1=Mark J. |last2=Johnson |first2=Christine |title=Occupational, industrial, and environmental toxicology|year=2003|publisher=Mosby|location=St Louis, Missouri|isbn=9780323013406|chapter-url=http://toxicology.ws/Greenberg/Chapter%2065%20-%20Residential%20Radon.pdf|edition=2nd|chapter=65 Residential Radon|editor1-first=Michael I. |editor1-last=Greenberg |editor2-first=Richard J. |editor2-last=Hamilton |editor3-first=Scott D. |editor3-last=Phillips |editor4-first=Gayla J. |editor4-last=McCluskey|access-date=28 November 2012}} |
|||
* {{cite web | url =http://www.units.miamioh.edu/ehso/radiationtraining/backgroundradiation/index.htm | title =Background Radiation & Other Sources of Exposure | website =Radiation Safety Training | publisher =[[Miami University]] | access-date =30 September 2016 }} |
|||
* {{cite web|title=Radiation Exposure During Commercial Airline Flights|access-date=2011-03-17|url=http://www.hps.org/publicinformation/ate/faqs/commercialflights.html}} |
|||
* {{cite web|url=http://www.hps.org/publicinformation/ate/faqs/commercialflights.html|title=Radiation exposure during commercial airline flights|author=Health Physics Society|access-date=2013-01-24}} |
|||
* {{cite web | url =https://www.faa.gov/data_research/research/med_humanfacs/aeromedical/radiobiology/ | title =Radiobiology Research Team | website =Federal Aviation Administration | access-date =23 January 2022}} |
|||
* {{cite web |url = http://sciencedemonstrations.fas.harvard.edu/icb/icb.do?keyword=k16940&pageid=icb.page102829&pageContentId=icb.pagecontent270775&state=maximize&view=view.do&viewParam_name=indepth.html#a_icb_pagecontent270775 |title = Radioactive human body – Harvard University Natural Science Lecture Demonstrations |archive-url=https://web.archive.org/web/20150612194741/http://sciencedemonstrations.fas.harvard.edu/icb/icb.do?keyword=k16940&pageid=icb.page102829&pageContentId=icb.pagecontent270775&state=maximize&view=view.do&viewParam_name=indepth.html#a_icb_pagecontent270775 |archive-date=12 June 2015 |url-status=dead}} |
|||
* {{cite web|url = http://www.ead.anl.gov/pub/doc/carbon14.pdf|title = Carbon 14|work = Human Health Fact Sheet|date = August 2005|publisher = Argonne National Lab|archive-url = https://web.archive.org/web/20080227103725/http://www.ead.anl.gov/pub/doc/carbon14.pdf|archive-date = 27 February 2008|access-date = 4 April 2011|url-status = live}} |
|||
* {{cite book |last=Asimov |first=Isaac |author-link=Isaac Asimov |title=Only A Trillion |orig-year=1957 |edition=Revised and updated |year=1976 |publisher=ACE books |location=New York |pages=37–39 |chapter=The Explosions Within Us |isbn= 978-1-157-09468-5 }} |
|||
{{refend}} |
{{refend}} |
||
== Спољашње везе == |
|||
| ⚫ | |||
{{Commons category|Becquerel}} |
|||
| ⚫ | |||
* [https://web.archive.org/web/20050316054529/http://www1.bipm.org/en/si/derived_units/2-2-2.html Derived units] on the [[International Bureau of Weights and Measures]] (BIPM) web site |
|||
{{СИ јединице}} |
{{СИ јединице}} |
||
Верзија на датум 8. јул 2022. у 01:07
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
| Бекерел | |
|---|---|
| Систем | Изведене јединице СИ система |
| Јединица | Активност |
| Симбол | Bq |
| Именован по | Анри Бекерел |
| Јединична претварања | |
| 1 Bq у ... | ... је једнак са ... |
| радерфорд | 10−6 Rd |
| кири | 2,703×10−11 Ci ≅ 27 pCi |
| СИ основне јединице | s−1 |
Бекерел (енгл. becquerel; симбол: Bq) изведена је јединица СИ система која се дефинише као активност количине радиоактивног материјала где се једно језгро распада у секунди.[1] Бекерел је, стога, еквивалентан s-1. Старија јединица радиоактивности је била кири (Ci), дефинисана као 37×109 бекерела или 37 GBq.[2]
Назван је по Анрију Бекерелу, који је делио Нобелову награду с Маријом Кири због њиховог рада у откривању радиоактивности.[3]
За фиксирану масу радиоактивног метала, број бекерела се мења временом. Под неким околностима, количине радиоактивног материјала су дате после неког времена за подешавање. На пример, може да се узме десетодневна подешена цифра, тј. количина радиоактивности која ће и даље бити присутна после десет дана. Ово склања нагласак са кратковековних изотопа.
Намеравало се да се бекерел користи у СИ-ју, уместо реципрочне секунде, као јединица мерења активности. Ово је посебно уведено због опасности по људско здравље које могу да проистекну услед грешака везане за реципрочну секунду. Користећи бекерел, активнији извор има већи број (па је и опасније). Користећи 1/s или s као секунду може да доведе до конфузије.
Дефиниција
1 Bq = 1 s−1
A special name was introduced for the reciprocal second (s−1) to represent radioactivity to avoid potentially dangerous mistakes with prefixes. For example, 1 µs−1 would mean 106 disintegrations per second: 1·(10−6 s)−1 = 106 s−1,[4] whereas 1 µBq would mean 1 disintegration per 1 million seconds. Other names considered were hertz (Hz), a special name already in use for the reciprocal second, and Fourier (Fr).[4] The hertz is now only used for periodic phenomena.[5] Whereas 1 Hz is 1 cycle per second, 1 Bq is 1 aperiodic radioactivity event per second.
The gray (Gy) and the becquerel (Bq) were introduced in 1975.[6] Between 1953 and 1975, absorbed dose was often measured in rads. Decay activity was measured in curies before 1946 and often in rutherfords between 1946[7] and 1975.
Велика слова и префикси јединица
As with every International System of Units (SI) unit named for a person, the first letter of its symbol is uppercase (Bq). However, when an SI unit is spelled out in English, it should always begin with a lowercase letter (becquerel)—except in a situation where any word in that position would be capitalized, such as at the beginning of a sentence or in material using title case.[8]
Like any SI unit, Bq can be prefixed; commonly used multiples are kBq (kilobecquerel, 103 Bq), MBq (megabecquerel, 106 Bq, equivalent to 1 rutherford), GBq (gigabecquerel, 109 Bq), TBq (terabecquerel, 1012 Bq), and PBq (petabecquerel, 1015 Bq). Large prefixes are common for practical uses of the unit.
Прорачун радиоактивности
For a given mass (in grams) of an isotope with atomic mass (in g/mol) and a half-life of (in s), the radioactivity can be calculated using:
With = 6,02214076×1023 mol-1, the Avogadro constant.
Since is the number of moles (), the amount of radioactivity can be calculated by:
For instance, on average each gram of potassium contains 117 micrograms of 40K (all other naturally occurring isotopes are stable) that has a of 1,277×109 years = 4,030×1016 s,[9] and has an atomic mass of 39.964 g/mol,[10] so the amount of radioactivity associated with a gram of potassium is 30 Bq.
Примери
For practical applications, 1 Bq is a small unit. For example, the roughly 0.0169 g of potassium-40 present in a typical human body produces approximately 4,400 disintegrations per second or 4.4 kBq of activity.[11]
The global inventory of carbon-14 is estimated to be 8,5×1018 Bq (8.5 EBq, 8.5 exabecquerel).[12] The nuclear explosion in Hiroshima (an explosion of 16 kt or 67 TJ) is estimated to have injected 8×1024 Bq (8 YBq, 8 yottabecquerel) of radioactive fission products into the atmosphere.[13]
These examples are useful for comparing the amount of activity of these radioactive materials but should not be confused with the amount of exposure to ionizing radiation that these materials represent. The level of exposure and thus the absorbed dose received are what should be considered when assessing the effects of ionizing radiation on humans.
Релација са киријом
The becquerel succeeded the curie (Ci),[14] an older, non-SI unit of radioactivity based on the activity of 1 gram of radium-226. The curie is defined as 3,7×1010 s−1, or 37 GBq.[4][15]
Conversion factors:
- 1 Ci = 3,7×1010 Bq = 37 GBq
- 1 μCi = 37,000 Bq = 37 kBq
- 1 Bq = 2,7×10−11 Ci = 2,7×10−5 µCi
- 1 MBq = 0.027 mCi
Однос према другим величинама у вези са зрачењем
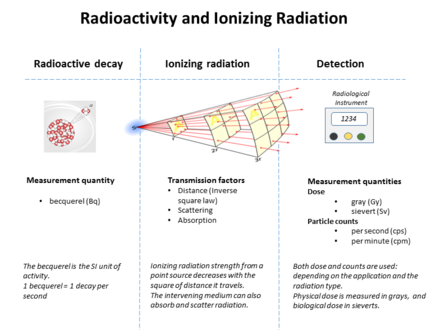
The following table shows radiation quantities in SI and non-SI units. WR (formerly 'Q' factor) is a factor that scales the biological effect for different types of radiation, relative to x-rays. (e.g. 1 for beta radiation, 20 for alpha radiation, and a complicated function of energy for neutrons) In general conversion between rates of emission, the density of radiation, the fraction absorbed, and the biological effects, requires knowledge of the geometry between source and target, the energy and the type of the radiation emitted, among other factors.[16]
Референце
- ^ „Radioactivity : Radioactive Activity Doses”. www.radioactivity.eu.com. Приступљено 20. 2. 2020.
- ^ „Radiation Protection Guidance For Hospital Staff – Stanford Environmental Health & Safety”. ehs.stanford.edu. Приступљено 20. 2. 2020.
- ^ „BIPM - Becquerel”. BIPM. Приступљено 2012-10-24.
- ^ а б в Allisy, A. (1995), „From the curie to the becquerel”, Metrologia, 32 (6): 467—479, Bibcode:1995Metro..31..467A, doi:10.1088/0026-1394/31/6/006
- ^ „BIPM - Table 3”. BIPM. Приступљено 2015-07-19. „(d) The hertz is used only for periodic phenomena, and the becquerel is used only for stochastic processes in activity referred to a radionuclide.”
- ^ Harder, D (1976), „[The new radiologic units of measurement gray and becquerel (author's translation from the German original)]”, Röntgen-Blätter, 29 (1): 49—52, PMID 1251122.
- ^ Lind, SC (1946), „New units for the measurement of radioactivity”, Science, 103 (2687): 761—762, Bibcode:1946Sci...103..761L, PMID 17836457, S2CID 5343688, doi:10.1126/science.103.2687.761-a.
- ^ „SI Brochure: The International System of Units (SI)”. SI Brochure (8 изд.). BIPM. 2014.
- ^ „Table of Isotopes decay data”. Lund University. 1990-06-01. Приступљено 2014-01-12.
- ^ „Atomic Weights and Isotopic Compositions for All Elements”. NIST. Приступљено 2014-01-12.
- ^ Radioactive human body — Harvard University Natural Science Lecture Demonstrations - Accessed October 2013
- ^ G.R. Choppin, J.O.Liljenzin, J. Rydberg, "Radiochemistry and Nuclear Chemistry", 3rd edition, Butterworth-Heinemann, 2002. ISBN 978-0-7506-7463-8.
- ^ Harrison (2013). Pollution : Causes, Effects and Control. Cambridge: Royal Society of Chemistry. ISBN 978-1-68015-810-6. OCLC 869096285.
- ^ It was adopted by the BIPM in 1975, see resolution 8 of the 15th CGPM meeting
- ^ Resolution 7 of the 12th CGPM Архивирано 2021-02-19 на сајту Wayback Machine (1964)
- ^ http://hps.org/publicinformation/ate/faqs/gammaandexposure.html
Литература
- E Richard Cohen; Tom Cvitas; Jeremy G Frey; Bertil Holstrom; John W Jost, ур. (2007). Quantities, Units and Symbols in Physical Chemistry (PDF). International Union of Pure and Applied Chemistry (3. изд.). Royal Society of Chemistry; 3rd edition. ISBN 0854044337.
- International Union of Pure and Applied Chemistry (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition, Oxford: Blackwell Science. ISBN 0-632-03583-8. Electronic version.
- International Atomic Energy Agency (2007). IAEA Safety Glossary: Terminology Used in Nuclear Safety and Radiation Protection. ISBN 9789201007070.
- United Nations Scientific Committee on the Effects of Atomic Radiation (2008). Sources and effects of ionizing radiation. New York: United Nations (објављено 2010). стр. 4. ISBN 978-92-1-142274-0. Приступљено 9. 11. 2012.
- Ionizing radiation exposure of the population of the United States. Bethesda, Md.: National Council on Radiation Protection and Measurements. 2009. ISBN 978-0-929600-98-7. NCRP No. 160. Архивирано из оригинала 2. 2. 2014. г. Приступљено 9. 11. 2012.
- Ministry of Education, Culture, Sports, Science, and Technology of Japan "Radiation in environment" Архивирано 22 март 2011 на сајту Wayback Machine
- „Naturally-Occurring Radioactive Materials (NORM)”. World Nuclear Association. март 2019.
- „Exposure to radiation from natural sources”. Nuclear Safety & Security. IAEA. Архивирано из оригинала 9. 2. 2016. г. Приступљено 4. 1. 2016.
- United Nations Scientific Committee on the Effects of Atomic Radiation (2006). „Annex E: Sources-to-effects assessment for radon in homes and workplaces” (PDF). Effects of Ionizing Radiation. II. New York: United Nations (објављено 2008). ISBN 978-92-1-142263-4. Приступљено 2. 12. 2012.
- „Radon and Cancer: Questions and Answers – National Cancer Institute (USA)”. 6. 12. 2011.
- Fornalski, K. W.; Adams, R.; Allison, W.; Corrice, L. E.; Cuttler, J. M.; Davey, Ch.; Dobrzyński, L.; Esposito, V. J.; Feinendegen, L. E.; Gomez, L. S.; Lewis, P.; Mahn, J.; Miller, M. L.; Pennington, Ch. W.; Sacks, B.; Sutou, S.; Welsh, J. S. (2015). „The assumption of radon-induced cancer risk”. Cancer Causes & Control. 10 (26): 1517—18. PMID 26223888. S2CID 15952263. doi:10.1007/s10552-015-0638-9.
- Thomas, John J.; Thomas, Barbara R.; Overeynder, Helen M. (27—30. 9. 1995). Indoor Radon Concentration Data: Its Geographic and Geologic Distribution, an Example from the Capital District, NY (PDF). International Radon Symposium. Nashville, TN: American Association of Radon Scientists and Technologists. Приступљено 2012-11-28.
- Upfal, Mark J.; Johnson, Christine (2003). „65 Residential Radon” (PDF). Ур.: Greenberg, Michael I.; Hamilton, Richard J.; Phillips, Scott D.; McCluskey, Gayla J. Occupational, industrial, and environmental toxicology (2nd изд.). St Louis, Missouri: Mosby. ISBN 9780323013406. Приступљено 28. 11. 2012.
- „Background Radiation & Other Sources of Exposure”. Radiation Safety Training. Miami University. Приступљено 30. 9. 2016.
- „Radiation Exposure During Commercial Airline Flights”. Приступљено 2011-03-17.
- Health Physics Society. „Radiation exposure during commercial airline flights”. Приступљено 2013-01-24.
- „Radiobiology Research Team”. Federal Aviation Administration. Приступљено 23. 1. 2022.
- „Radioactive human body – Harvard University Natural Science Lecture Demonstrations”. Архивирано из оригинала 12. 6. 2015. г.
- „Carbon 14” (PDF). Human Health Fact Sheet. Argonne National Lab. август 2005. Архивирано (PDF) из оригинала 27. 2. 2008. г. Приступљено 4. 4. 2011.
- Asimov, Isaac (1976) [1957]. „The Explosions Within Us”. Only A Trillion (Revised and updated изд.). New York: ACE books. стр. 37—39. ISBN 978-1-157-09468-5.
Спољашње везе
- Derived units on the International Bureau of Weights and Measures (BIPM) web site










