Metabolizam gvožđa kod čoveka — разлика између измена
. |
(нема разлике)
|
Верзија на датум 10. мај 2018. у 22:29
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
Metabolizam gvožđa kod čoveka je set hemijskih reakcija koje održavaju ljudsku homeostazu gvožđa na sistemskom i ćelijskom nivou. Iron is both necessary to the body and potentially toxic, and controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron deficiency anemia.
Značaj regulacije gvožđa
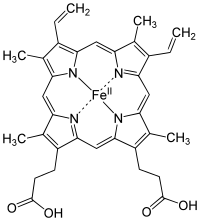
Gvožđe je esencijalni bioelement za većinu formi života, od bakterija do sisara. Its importance lies in its ability to mediate electron transfer. In the ferrous state, iron acts as an electron donor, while in the ferric state it acts as an acceptor. Thus, iron plays a vital role in the catalysis of enzymatic reactions that involve electron transfer (reduction and oxidation, redox). Proteins can contain iron as part of different cofactors, such as iron-sulfur clusters (Fe-S) and heme groups, both of which are assembled in mitochondria.
Ćelijska respiracija
Human cells require iron in order to obtain energy as ATP from a multi-step process known as cellular respiration, more specifically from oxidative phosphorylation at the mitochondrial cristae. Iron is present in the iron-sulfur clusters and heme groups of the electron transport chain proteins that generate a proton gradient that allows ATP synthase to synthesize ATP (chemiosmosis).
Heme groups are part of hemoglobin, a protein found in red blood cells that serves to transport oxygen from the lungs to the tissues. Heme groups are also present in myoglobin to store and diffuse oxygen in muscle cells.
Kiseonikčni transport
The human body needs iron for oxygen transport. Oxygen (O2) is required for the functioning and survival of nearly all cell types. Oxygen is transported from the lungs to the rest of the body bound to the heme group of hemoglobin in erythrocytes. In muscles cells, iron binds myoglobin, which regulates its release.
Toksičnost
Iron is also potentially toxic. Its ability to donate and accept electrons means that it can catalyze the conversion of hydrogen peroxide into free radicals. Free radicals can cause damage to a wide variety of cellular structures, and ultimately kill the cell.[1]
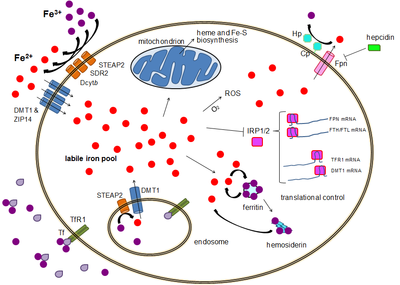
Iron bound to proteins or cofactors such as heme is safe. Also, there are virtually no truly free iron ions in the cell, since they readily form complexes with organic molecules. However, some of the intracellular iron is bound to low-affinity complexes, and is termed labile iron or "free" iron. Iron in such complexes can cause damage as described above.[2]
To prevent that kind of damage, all life forms that use iron bind the iron atoms to proteins. This binding allows cells to benefit from iron while also limiting its ability to do harm.[1][3] Typical intracellular labile iron concentrations in bacteria are 10-20 micromolar,[4] though they can be 10-fold higher in anaerobic environment,[5] where free radicals and reactive oxygen species are scarcer. In mammalian cells, intracellular labile iron concentrations are typically smaller than 1 micromolar, less than 5 percent of total cellular iron.[2]
Reference
- ^ а б Conrad ME, Umbreit JN (април 2000). „Disorders of iron metabolism”. The New England Journal of Medicine. 342 (17): 1293—4. PMID 10787338. doi:10.1056/NEJM200004273421716.
- ^ а б Kakhlon O, Cabantchik ZI (2002). „The labile iron pool: characterization, measurement, and participation in cellular processes”. Free Radical Biology and Medicine. 33 (8): 1037—1046. doi:10.1016/s0891-5849(02)01006-7.
- ^ Andrews NC (децембар 1999). „Disorders of iron metabolism”. The New England Journal of Medicine. 341 (26): 1986—95. PMID 10607817. doi:10.1056/NEJM199912233412607.
- ^ Yan Y, Waite-Cusic JG, Kuppusamy P, Yousef AE (јануар 2013). „Intracellular free iron and its potential role in ultrahigh-pressure-induced inactivation of Escherichia coli”. Applied and Environmental Microbiology. 79 (2): 722—724. PMC 3553779
 . PMID 23124235. doi:10.1128/aem.02202-12.
. PMID 23124235. doi:10.1128/aem.02202-12.
- ^ Yamamoto Y, Fukui K, Koujin N, Ohya H, Kimura K, Kamio Y (2004). „Regulation of the intracellular free iron pool by Dpr provides oxygen tolerance to Streptococcus mutans.”. Journal of Bacteriology. 186 (18): 5997—6002. PMC 515136
 . PMID 15342568. doi:10.1128/jb.186.18.5997-6002.2004.
. PMID 15342568. doi:10.1128/jb.186.18.5997-6002.2004.
Literatura
- Andrews S, Norton I, Salunkhe AS, Goodluck H, Aly WS, Mourad-Agha H, Cornelis P (2013). „Chapter 7, Control of Iron Metabolism in Bacteria”. Ур.: Banci L. Metallomics and the Cell. Metal Ions in Life Sciences. 12. Springer. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-1_7. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- Andrews NC (мај 2004). „Anemia of inflammation: the cytokine-hepcidin link”. The Journal of Clinical Investigation. 113 (9): 1251—3. PMC 398435
 . PMID 15124013. doi:10.1172/JCI21441.
. PMID 15124013. doi:10.1172/JCI21441. - Camaschella C (децембар 2005). „Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders”. Blood. 106 (12): 3710—7. PMID 16030190. doi:10.1182/blood-2005-05-1857.
- Frazer DM, Anderson GJ (октобар 2005). „Iron imports. I. Intestinal iron absorption and its regulation”. American Journal of Physiology. Gastrointestinal and Liver Physiology. 289 (4): G631—5. PMID 16160078. doi:10.1152/ajpgi.00220.2005.
- Insel P, Ross D, McMahon K, Bernstein M (2011). „Iron”. Nutrition (4th изд.). Sudbury, Massachusetts: Jones and Bartlett Publishers. стр. 510—514. ISBN 978-0-7637-7663-3. Приступљено 25. 6. 2012. See esp. pp. 513-514
- Lammi-Keef CJ, Couch SC, Philipson EH, ур. (2008). „Dietary diversification and modification of iron”. Handbook of Nutrition and Pregnancy. Nutrition & Health. Totowa, New Jersey: Humana Press. стр. 350—351. ISBN 978-1-59745-112-3. doi:10.1007/978-1-59745-112-3. Приступљено 25. 6. 2012.
- Panel on Micronutrients; Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes; the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (2001). „Iron”. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C: Food and Nutrition Board, Institute of Medicine. стр. 290—393. ISBN 978-0-309-07279-3. Приступљено 25. 6. 2012.
- Reilly C (2004). „Iron”. The Nutritional Trace Metals. Oxford, UK & Ames, Iowa: Blackwell Publishing. стр. 35—81. ISBN 1-4051-1040-6. Приступљено 25. 6. 2012.
