Минералогија — разлика између измена
м уклоњена категорија Минералози помоћу геџета HotCat ознака: ручно враћање |
. ознака: везе до вишезначних одредница |
||
| Ред 1: | Ред 1: | ||
{{short description|Научно проучавање минерала и минерализованих артефаката}} |
|||
[[Датотека:Mineralogy between its other sciences around.png|мини|Минералогија је мешавина [[хемија|хемије]], [[Nauka o materijalima|науке о материјалима]], [[физика|физике]] и [[геологија|геологије]]]] |
[[Датотека:Mineralogy between its other sciences around.png|мини|Минералогија је мешавина [[хемија|хемије]], [[Nauka o materijalima|науке о материјалима]], [[физика|физике]] и [[геологија|геологије]]]] |
||
'''Минералогија''' је једна од [[Науке о Земљи|наука о Земљи]] која се бави изучавањем [[минерал]]а, њиховог [[хемија|хемијског]] састава, [[кристал]]не структуре и [[физика|физичких]] својстава. Посебне дисциплине у минералогији изучавају процесе настанка минерала и појавне облике, баве се класификацијом минерала, њиховом [[географија|географском]] дистрибуцијом (распрострањењем) као и могућностима употребе. Минералогијом се баве минералози. |
'''Минералогија''' је једна од [[Науке о Земљи|наука о Земљи]] која се бави изучавањем [[минерал]]а, њиховог [[хемија|хемијског]] састава, [[кристал]]не структуре и [[физика|физичких]] својстава.<ref>{{cite encyclopedia|title=Mineralogy|encyclopedia=American Heritage Dictionary |url=https://www.ahdictionary.com/word/search.html?q=mineralogy |publisher=Houghton Mifflin Harcourt Publishing Company|date=2017 |access-date=19 October 2017}}</ref><ref name=collins>{{cite encyclopedia|title=Mineralogy |encyclopedia=Collins English Dictionary |publisher=HarperCollins Publishers|url=https://www.collinsdictionary.com/dictionary/english/mineralogy|access-date=19 October 2017 }}</ref> Посебне дисциплине у минералогији изучавају процесе настанка минерала и појавне облике, баве се класификацијом минерала, њиховом [[географија|географском]] дистрибуцијом (распрострањењем) као и могућностима употребе. Минералогијом се баве минералози. |
||
== Историјски развој == |
== Историјски развој == |
||
{{rut}} |
|||
| ⚫ | О најранијим познатим изучавањима, претпоставкама и теорији минералогије пронађени су записи из античке [[Вавилонија|Вавилоније]], потом античког грчко-римског периода, античког и доба средњег века Кине, и записи ''прана'' на [[санскрт|санскриту]] из древне Индије |
||
[[File:Mohs mineralogy vol 2 plate 19.jpg|thumb|250px|right|Page from ''Treatise on mineralogy'' by [[Friedrich Mohs]] (1825)]] |
|||
[[File:Moon Mineralogy Mapper left.jpg|thumb|right|250px|The [[Moon Mineralogy Mapper]], a [[spectrometer]] that mapped the lunar surface<ref name="JPL M3">{{cite web|url =http://jpl.nasa.gov/news/news.cfm?release=2008-239 |title =NASA Instrument Inaugurates 3-D Moon Imaging |access-date =19 December 2008 |publisher =JPL}}</ref>]] |
|||
| ⚫ | О најранијим познатим изучавањима, претпоставкама и теорији минералогије пронађени су записи из античке [[Вавилонија|Вавилоније]], потом античког грчко-римског периода, античког и доба средњег века Кине, и записи ''прана'' на [[санскрт|санскриту]] из древне Индије,<ref name="needham volume 3 637">Needham, Volume 3, 637.</ref> као и у делима древног исламског света.<ref name=Needham>{{cite book|last=Needham|first=Joseph|title=Science and civilisation in China|url=https://archive.org/details/sciencecivilisat00need_228|url-access=limited|year=1959|publisher=Cambridge University Press|location=Cambridge |isbn=978-0521058018|pages=[https://archive.org/details/sciencecivilisat00need_228/page/n749 637]–638}}</ref> Ипак, прве систематске научне студије минерала и стена јавиле су се тек у Европи у периоду након ренесансе.<ref name="needham volume 3 636">Needham, Volume 3, 636.</ref> Оправданост и потврде изучавања минералогије успостављени су на основама кристалографије и микроскопских изучавања стена (препарата) са појавом [[микроскоп]]а у 17. веку.<ref name="needham volume 3 636"/> |
||
[[Nicholas Steno]] first observed the law of constancy of interfacial angles (also known as the first law of crystallography) in quartz crystals in 1669.<ref name=Ness/>{{rp|4}} This was later generalized and established experimentally by [[Jean-Baptiste L. Romé de l'Isle]]e in 1783.<ref>{{cite encyclopedia|title=Law of the constancy of interfacial angles|encyclopedia=Online dictionary of crystallography|date=24 August 2014 |publisher=International Union of Crystallography|url=http://reference.iucr.org/dictionary/Law_of_the_constancy_of_interfacial_angles|access-date=22 September 2015}}</ref> [[René Just Haüy]], the "father of modern crystallography", showed that crystals are periodic and established that the orientations of crystal faces can be expressed in terms of rational numbers, as later encoded in the Miller indices.<ref name=Ness/>{{rp|4}} In 1814, [[Jöns Jacob Berzelius]] introduced a classification of minerals based on their chemistry rather than their crystal structure.<ref name=Rafferty>{{cite book|last1=Rafferty|first1=John P.|title=Geological sciences|date=2012|publisher=Britannica Educational Pub. in association with Rosen Educational Services|location=New York|isbn=9781615304950|pages=14–15|edition=1st}}</ref> [[William Nicol (geologist)|William Nicol]] developed the [[Nicol prism]], which polarizes light, in 1827–1828 while studying fossilized wood; [[Henry Clifton Sorby]] showed that thin sections of minerals could be identified by their optical properties using a [[polarizing microscope]].<ref name=Ness/>{{rp|4}}<ref name=Rafferty/>{{rp|15}} [[James D. Dana]] published his first edition of ''A System of Mineralogy'' in 1837, and in a later edition introduced a chemical classification that is still the standard.<ref name=Ness/>{{rp|4}}<ref name=Rafferty/>{{rp|15}} X-ray diffraction was demonstrated by [[Max von Laue]] in 1912, and developed into a tool for analyzing the crystal structure of minerals by the father/son team of [[William Henry Bragg]] and [[William Lawrence Bragg]].<ref name=Ness/>{{rp|4}} |
|||
== Физичка минералогија == |
== Физичка минералогија == |
||
[[File:Calcit Scalenoeder - Egremont, England.jpg|thumb|250px|left|[[Calcite]] is a [[carbonate minerals|carbonate mineral]] (CaCO<sub>3</sub>) with a [[rhombohedral]] crystal structure.]] |
|||
Физичка минералогија изучава физичке особине минерала. Описом физичких карактеристика најлакше се идентификују, класификују и категоришу минерали. |
|||
[[File:Aragonite redbrown crystals.jpg|thumb|250px|left|[[Aragonite]] is an [[orthorhombic]] polymorph of calcite.]] |
|||
Физичка минералогија изучава физичке особине минерала. Описом физичких карактеристика најлакше се идентификују, класификују и категоришу минерали. При том се између осталог узимају у обзир: [[Кристалне системе|кристална система]], [[облик кристала]], [[ближњење]], [[цепљивост и прелом]], [[сјајност]], боја, [[огреб]], [[тврдоћа]], [[густина минерала]], magnetic and electric properties; radioactivity and solubility in [[hydrogen chloride]] ({{Chemical formula|H||Cl|}}).<ref name=Ness>{{cite book|last1=Nesse|first1=William D.|title=Introduction to mineralogy|date=2012|publisher=Oxford University Press|location=New York|isbn=978-0199827381|edition=2nd}}</ref>{{rp|97–113}}<ref name=Klein>{{cite book|last1=Klein|first1=Cornelis |first2=Anthony R. |last2=Philpotts|title=Earth materials : introduction to mineralogy and petrology|date=2013|publisher=Cambridge University Press|location=New York|isbn=9780521145213}}</ref>{{rp|39–53}} |
|||
* [[Кристалне системе|Кристална система]] |
|||
* [[Облик кристала]] |
|||
''Hardness'' is determined by comparison with other minerals. In the [[Mohs scale of mineral hardness|Mohs scale]], a standard set of minerals are numbered in order of increasing hardness from 1 (talc) to 10 (diamond). A harder mineral will scratch a softer, so an unknown mineral can be placed in this scale, by which minerals; it scratches and which scratch it. A few minerals such as [[calcite]] and [[kyanite]] have a hardness that depends significantly on direction.<ref name=Manual/>{{rp|254–255}} Hardness can also be measured on an absolute scale using a [[sclerometer]]; compared to the absolute scale, the Mohs scale is nonlinear.<ref name=Klein/>{{rp|52}} |
|||
* [[Ближњење]] |
|||
* [[Цепљивост и прелом]] |
|||
''Tenacity'' refers to the way a mineral behaves, when it is broken, crushed, bent or torn. A mineral can be [[brittle]], [[malleable]], [[sectile]], [[ductile]], [[Stiffness|flexible]] or [[elastic deformation|elastic]]. An important influence on tenacity is the type of chemical bond (''e.g.,'' [[ionic bonding|ionic]] or [[metallic bonding|metallic]]).<ref name=Manual/>{{rp|255–256}} |
|||
* [[Сјајност]] |
|||
* Боја |
|||
Of the other measures of mechanical cohesion, ''cleavage'' is the tendency to break along certain crystallographic planes. It is described by the quality (''e.g.'', perfect or fair) and the orientation of the plane in crystallographic nomenclature. |
|||
* [[Огреб]] |
|||
* [[Тврдина]] |
|||
''Parting'' is the tendency to break along planes of weakness due to pressure, twinning or [[exsolution]]. Where these two kinds of break do not occur, ''fracture'' is a less orderly form that may be ''[[Conchoidal fracture|conchoidal]]'' (having smooth curves resembling the interior of a shell), ''fibrous'', ''splintery'', ''hackly'' (jagged with sharp edges), or ''uneven''.<ref name="Manual" />{{rp|253–254}} |
|||
* [[Густина минерала]] |
|||
If the mineral is well crystallized, it will also have a distinctive [[crystal habit]] (for example, hexagonal, columnar, [[botryoidal]]) that reflects the [[crystal structure]] or internal arrangement of atoms.<ref name=Klein/>{{rp|40–41}} It is also affected by crystal defects and [[Crystal twinning|twinning]]. Many crystals are [[polymorphism (materials science)|polymorphic]], having more than one possible crystal structure depending on factors such as pressure and temperature.<ref name=Ness/>{{rp|66–68}}<ref name=Klein/>{{rp|126}} |
|||
== Кристална структура == |
|||
[[File:Perovskite.jpg|thumb|250px|The [[perovskite structure|perovskite crystal structure]]. The most abundant mineral in the Earth, [[bridgmanite]], has this structure.<ref name=Sharp>{{cite journal|last1=Sharp|first1=T.|title=Bridgmanite – named at last|journal=Science|date=27 November 2014|volume=346|issue=6213|pages=1057–1058|doi=10.1126/science.1261887|pmid=25430755|s2cid=206563252}}</ref> Its chemical formula is (Mg,Fe)SiO<sub>3</sub>; the red spheres are oxygen, the blue spheres silicon and the green spheres magnesium or iron.]] |
|||
{{main article|Кристална структура}} |
|||
{{See also|Кристалографија}} |
|||
The crystal structure is the arrangement of atoms in a crystal. It is represented by a [[Crystal lattice|lattice]] of points which repeats a basic pattern, called a [[unit cell]], in three dimensions. The lattice can be characterized by its symmetries and by the dimensions of the unit cell. These dimensions are represented by three ''[[Miller index|Miller indices]]''.<ref name=Ashcroft>{{cite book|last1=Ashcroft|first1=Neil W.|last2=Mermin|first2=N. David|title=Solid state physics|date=1977|publisher=Holt, Rinehart and Winston|location=New York|isbn=9780030839931|edition=27. repr.|url-access=registration|url=https://archive.org/details/solidstatephysic00ashc}}</ref>{{rp|91–92}} The lattice remains unchanged by certain symmetry operations about any given point in the lattice: [[Reflection symmetry|reflection]], [[Rotational symmetry|rotation]], [[Point reflection|inversion]], and [[Improper rotation|rotary inversion]], a combination of rotation and reflection. Together, they make up a mathematical object called a ''[[crystallographic point group]]'' or ''crystal class''. There are 32 possible crystal classes. In addition, there are operations that displace all the points: [[Translational symmetry|translation]], [[screw axis]], and [[glide plane]]. In combination with the point symmetries, they form 230 possible [[space group]]s.<ref name=Ashcroft/>{{rp|125–126}} |
|||
Most geology departments have [[X-ray]] [[powder diffraction]] equipment to analyze the crystal structures of minerals.<ref name=Klein/>{{rp|54–55}} X-rays have wavelengths that are the same order of magnitude as the distances between atoms. [[Diffraction]], the constructive and destructive interference between waves scattered at different atoms, leads to distinctive patterns of high and low intensity that depend on the geometry of the crystal. In a sample that is ground to a powder, the X-rays sample a random distribution of all crystal orientations.<ref name=Dinnebier>{{cite book|last1=Dinnebier|first1=Robert E.|last2=Billinge|first2=Simon J.L.|chapter=1. Principles of powder diffraction|editor-last1=Dinnebier|editor-first1=Robert E.|editor-last2=Billinge|editor-first2=Simon J.L.|title=Powder diffraction : theory and practice|url=https://archive.org/details/powderdiffractio00redi|url-access=limited|date=2008|publisher=Royal Society of Chemistry|location=Cambridge|isbn=9780854042319|pages=[https://archive.org/details/powderdiffractio00redi/page/n23 1]–19|edition=Repr.}}</ref> Powder diffraction can distinguish between minerals that may appear the same in a hand sample, for example [[quartz]] and its polymorphs [[tridymite]] and [[cristobalite]].<ref name=Klein/>{{rp|54}} |
|||
[[Isomorphism (crystallography)|Isomorphous]] minerals of different compositions have similar powder diffraction patterns, the main difference being in spacing and intensity of lines. For example, the {{Chemical formula|Na||Cl}} ([[halite]]) crystal structure is space group ''Fm3m''; this structure is shared by [[sylvite]] ({{Chemical formula|K||Cl}}), [[periclase]] ({{Chemical formula|Mg||O}}), [[bunsenite]] ({{Chemical formula|Ni||O}}), [[galena]] ({{Chemical formula|Pb||S}}), [[alabandite]] ({{Chemical formula|Mn||S}}), [[chlorargyrite]] ({{Chemical formula|Ag||Cl}}), and [[Titanium nitride|osbornite]] ({{Chemical formula|Ti||N}}).<ref name=Manual>{{cite book|last1=Klein|first1=Cornelis|last2=Hurlbut, Jr.|first2=Cornelius S.|title=Manual of mineralogy : (after James D. Dana)|date=1993|publisher=Wiley|location=New York|isbn=047157452X|edition=21st}}</ref>{{rp|150–151}} |
|||
== Хемијски елементи == |
|||
{{See also|Аналитичка хемија}} |
|||
[[File:Portable Micro-X-ray fluorescence machine.jpg|thumb|250px|Portable Micro-X-ray fluorescence machine]] |
|||
A few minerals are [[chemical element]]s, including [[sulfur]], [[copper]], [[silver]], and [[gold]], but the vast majority are [[Chemical compound|compounds]]. The classical method for identifying composition is ''[[Wet chemistry|wet chemical analysis]]'', which involves dissolving a mineral in an acid such as [[hydrochloric acid]] ({{Chemical formula|H||Cl|}}). The elements in solution are then identified using [[Colorimetry (chemical method)|colorimetry]], [[Titration|volumetric analysis]] or [[gravimetric analysis]].<ref name=Manual/>{{rp|224–225}} |
|||
Since 1960, most chemistry analysis is done using instruments. One of these, [[atomic absorption spectroscopy]], is similar to wet chemistry in that the sample must still be dissolved, but it is much faster and cheaper. The solution is vaporized and its absorption spectrum is measured in the visible and ultraviolet range.<ref name=Manual/>{{rp|225–226}} Other techniques are [[X-ray fluorescence]], [[electron microprobe]] analysis [[atom probe]] tomography and [[Atomic emission spectroscopy|optical emission spectrography]].<ref name=Manual/>{{rp|227–232}} |
|||
== Види још == |
== Види још == |
||
| Ред 23: | Ред 53: | ||
== Референце == |
== Референце == |
||
{{reflist}} |
{{reflist}} |
||
== Литература == |
|||
{{Refbegin|30em}} |
|||
*{{cite book|last1=Gribble|first1=C.D.|last2=Hall|first2=A.J.|title=Optical Mineralogy: Principles And Practice.|date=1993|publisher=CRC Press|location=London|isbn=9780203498705}} |
|||
*{{cite encyclopedia|first1=James A.|last1=Harrell|chapter=Mineralogy|editor-last1=Bagnall|editor-first1=Roger S.|editor-last2=Brodersen|editor-first2=Kai|editor-last3=Champion|editor-first3=Craige B.|editor-last4=Erskine|editor-first4=Andrew|encyclopedia=The encyclopedia of ancient history|date=2012|publisher=Wiley-Blackwell|location=Malden, MA|isbn=9781444338386|doi=10.1002/9781444338386.wbeah21217}} |
|||
*{{cite journal|last1=Hazen|first1=Robert M.|title=Mineralogy: A historical review|journal=Journal of Geological Education|date=1984|volume=32|issue=5|pages=288–298|url=https://hazen.carnegiescience.edu/sites/hazen.gl.ciw.edu/files/516-MinHist-JGE-1984.pdf|access-date=27 September 2017|doi=10.5408/0022-1368-32.5.288}} |
|||
*{{cite book|last1=Laudan|first1=Rachel|title=From mineralogy to geology : the foundations of a science, 1650-1830|date=1993|publisher=University of Chicago Press|location=Chicago|isbn=9780226469478|edition=Pbk.}} |
|||
*{{cite book|last1=Oldroyd|first1=David|title=Sciences of the earth : studies in the history of mineralogy and geology|date=1998|publisher=Ashgate|location=Aldershot|isbn=9780860787709}} |
|||
*{{cite book|last1=Perkins|first1=Dexter|title=Mineralogy|date=2014|publisher=Pearson Higher Ed|isbn=9780321986573}} |
|||
*{{cite book|last1=Rapp|first1=George R.|title=Archaeomineralogy|date=2002|publisher=Springer Berlin Heidelberg|location=Berlin, Heidelberg|isbn=9783662050057}} |
|||
*{{cite book|last1=Tisljar|first1=S.K. Haldar, Josip|title=Introduction to mineralogy and petrology|date=2013|publisher=Elsevier Science|location=Burlington|isbn=9780124167100}} |
|||
*{{cite book|last1=Wenk|first1=Hans-Rudolf|last2=Bulakh|first2=Andrey|title=Minerals: Their Constitution and Origin|date=2016|publisher=Cambridge University Press|isbn=9781316425282}} |
|||
*{{cite book|chapter=Book XV. History of Mineralogy|last1=Whewell|first1=William|title=History of the Inductive Sciences: From the Earliest to the Present Times|date=2010|publisher=Cambridge University Press|isbn=9781108019262|pages=187–252}} |
|||
*Bandy, Mark Chance and Jean A. Bandy (1955). ''De Natura Fossilium''. New York: George Banta Publishing Company. |
|||
*Chan, Alan Kam-leung and Gregory K. Clancey, Hui-Chieh Loy (2002).'' Historical Perspectives on East Asian Science, Technology and Medicine''. Singapore: Singapore University Press {{ISBN|9971-69-259-7}} |
|||
*[[Robert Hazen|Hazen, Robert M.]] (1984). "[https://hazen.carnegiescience.edu/sites/hazen.gl.ciw.edu/files/516-MinHist-JGE-1984.pdf Mineralogy: A historical review]". ''Journal of Geological Education'', '''32''', 288–298. |
|||
*Needham, Joseph (1986). ''Science and Civilization in China: Volume 3''. Taipei: Caves Books, Ltd. |
|||
*Povarennykh A.S. (1972) "A Short History of Mineralogy and the Classification of Minerals". Crystal Chemical Classification of Minerals, 3–26. Springer, Boston, MA. {{ISBN|978-1-4684-1743-2}} |
|||
*Ramsdell, Lewis S. (1963). ''Encyclopedia Americana: International Edition: Volume 19''. New York: Americana Corporation. |
|||
*Sivin, Nathan (1995). ''Science in Ancient China''. Brookfield, Vermont: VARIORUM, Ashgate Publishing. |
|||
{{Refend}} |
|||
== Спољашње везе == |
== Спољашње везе == |
||
{{Commonscat|Mineralogy}} |
{{Commonscat|Mineralogy}} |
||
* [http://www.mineralogy.eu/ The Virtual Museum of the History of Mineralogy] |
|||
* [http://www.mineralogy.eu// Virtual Museum of the History of Mineralogy] |
|||
* [https://web.archive.org/web/20140809003823/http://www.farlang.com/gemstones/agricola_textbook_of_mineralogy/page_001 Georg Agricola's "Textbook on Mineralogy" on gemstones and minerals] |
|||
=== Асоцијације === |
|||
{{colbegin|colwidth=20em}} |
|||
* [http://www.amfed.org/ American Federation of Mineral Societies] |
|||
* [http://sfmc-fr.org/ French Society of Mineralogy and Crystallography] |
|||
* [https://web.archive.org/web/20111029134635/http://www.geosociety.org/ Geological Society of America] |
|||
* [http://www.dmg-home.org/ German Mineralogical Society] |
|||
* [http://wwwobs.univ-bpclermont.fr/ima/ International Mineralogical Association] |
|||
* [http://www.socminpet.it/ Italian Mineralogical and Petrological Society] |
|||
* [http://mineralogicalassociation.ca/ Mineralogical Association of Canada] |
|||
* [http://www.minersoc.org/ Mineralogical Society of Great Britain and Ireland] |
|||
* [http://www.minsocam.org/ Mineralogical Society of America] |
|||
{{colend}} |
|||
{{Геологија}} |
{{Геологија}} |
||
{{Authority control}} |
|||
{{клица-минералогија}} |
|||
[[Категорија:Минералогија]] |
[[Категорија:Минералогија]] |
||
Верзија на датум 23. новембар 2021. у 21:13

Минералогија је једна од наука о Земљи која се бави изучавањем минерала, њиховог хемијског састава, кристалне структуре и физичких својстава.[1][2] Посебне дисциплине у минералогији изучавају процесе настанка минерала и појавне облике, баве се класификацијом минерала, њиховом географском дистрибуцијом (распрострањењем) као и могућностима употребе. Минералогијом се баве минералози.
Историјски развој
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|


О најранијим познатим изучавањима, претпоставкама и теорији минералогије пронађени су записи из античке Вавилоније, потом античког грчко-римског периода, античког и доба средњег века Кине, и записи прана на санскриту из древне Индије,[4] као и у делима древног исламског света.[5] Ипак, прве систематске научне студије минерала и стена јавиле су се тек у Европи у периоду након ренесансе.[6] Оправданост и потврде изучавања минералогије успостављени су на основама кристалографије и микроскопских изучавања стена (препарата) са појавом микроскопа у 17. веку.[6]
Nicholas Steno first observed the law of constancy of interfacial angles (also known as the first law of crystallography) in quartz crystals in 1669.[7]:4 This was later generalized and established experimentally by Jean-Baptiste L. Romé de l'Islee in 1783.[8] René Just Haüy, the "father of modern crystallography", showed that crystals are periodic and established that the orientations of crystal faces can be expressed in terms of rational numbers, as later encoded in the Miller indices.[7]:4 In 1814, Jöns Jacob Berzelius introduced a classification of minerals based on their chemistry rather than their crystal structure.[9] William Nicol developed the Nicol prism, which polarizes light, in 1827–1828 while studying fossilized wood; Henry Clifton Sorby showed that thin sections of minerals could be identified by their optical properties using a polarizing microscope.[7]:4[9]:15 James D. Dana published his first edition of A System of Mineralogy in 1837, and in a later edition introduced a chemical classification that is still the standard.[7]:4[9]:15 X-ray diffraction was demonstrated by Max von Laue in 1912, and developed into a tool for analyzing the crystal structure of minerals by the father/son team of William Henry Bragg and William Lawrence Bragg.[7]:4
Физичка минералогија


Физичка минералогија изучава физичке особине минерала. Описом физичких карактеристика најлакше се идентификују, класификују и категоришу минерали. При том се између осталог узимају у обзир: кристална система, облик кристала, ближњење, цепљивост и прелом, сјајност, боја, огреб, тврдоћа, густина минерала, magnetic and electric properties; radioactivity and solubility in hydrogen chloride (HCl).[7]:97–113[10]:39–53
Hardness is determined by comparison with other minerals. In the Mohs scale, a standard set of minerals are numbered in order of increasing hardness from 1 (talc) to 10 (diamond). A harder mineral will scratch a softer, so an unknown mineral can be placed in this scale, by which minerals; it scratches and which scratch it. A few minerals such as calcite and kyanite have a hardness that depends significantly on direction.[11]:254–255 Hardness can also be measured on an absolute scale using a sclerometer; compared to the absolute scale, the Mohs scale is nonlinear.[10]:52
Tenacity refers to the way a mineral behaves, when it is broken, crushed, bent or torn. A mineral can be brittle, malleable, sectile, ductile, flexible or elastic. An important influence on tenacity is the type of chemical bond (e.g., ionic or metallic).[11]:255–256
Of the other measures of mechanical cohesion, cleavage is the tendency to break along certain crystallographic planes. It is described by the quality (e.g., perfect or fair) and the orientation of the plane in crystallographic nomenclature.
Parting is the tendency to break along planes of weakness due to pressure, twinning or exsolution. Where these two kinds of break do not occur, fracture is a less orderly form that may be conchoidal (having smooth curves resembling the interior of a shell), fibrous, splintery, hackly (jagged with sharp edges), or uneven.[11]:253–254
If the mineral is well crystallized, it will also have a distinctive crystal habit (for example, hexagonal, columnar, botryoidal) that reflects the crystal structure or internal arrangement of atoms.[10]:40–41 It is also affected by crystal defects and twinning. Many crystals are polymorphic, having more than one possible crystal structure depending on factors such as pressure and temperature.[7]:66–68[10]:126
Кристална структура
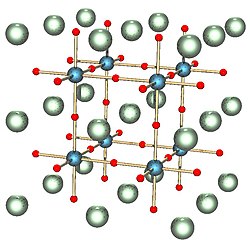
The crystal structure is the arrangement of atoms in a crystal. It is represented by a lattice of points which repeats a basic pattern, called a unit cell, in three dimensions. The lattice can be characterized by its symmetries and by the dimensions of the unit cell. These dimensions are represented by three Miller indices.[13]:91–92 The lattice remains unchanged by certain symmetry operations about any given point in the lattice: reflection, rotation, inversion, and rotary inversion, a combination of rotation and reflection. Together, they make up a mathematical object called a crystallographic point group or crystal class. There are 32 possible crystal classes. In addition, there are operations that displace all the points: translation, screw axis, and glide plane. In combination with the point symmetries, they form 230 possible space groups.[13]:125–126
Most geology departments have X-ray powder diffraction equipment to analyze the crystal structures of minerals.[10]:54–55 X-rays have wavelengths that are the same order of magnitude as the distances between atoms. Diffraction, the constructive and destructive interference between waves scattered at different atoms, leads to distinctive patterns of high and low intensity that depend on the geometry of the crystal. In a sample that is ground to a powder, the X-rays sample a random distribution of all crystal orientations.[14] Powder diffraction can distinguish between minerals that may appear the same in a hand sample, for example quartz and its polymorphs tridymite and cristobalite.[10]:54
Isomorphous minerals of different compositions have similar powder diffraction patterns, the main difference being in spacing and intensity of lines. For example, the NaCl (halite) crystal structure is space group Fm3m; this structure is shared by sylvite (KCl), periclase (MgO), bunsenite (NiO), galena (PbS), alabandite (MnS), chlorargyrite (AgCl), and osbornite (TiN).[11]:150–151
Хемијски елементи

A few minerals are chemical elements, including sulfur, copper, silver, and gold, but the vast majority are compounds. The classical method for identifying composition is wet chemical analysis, which involves dissolving a mineral in an acid such as hydrochloric acid (HCl). The elements in solution are then identified using colorimetry, volumetric analysis or gravimetric analysis.[11]:224–225
Since 1960, most chemistry analysis is done using instruments. One of these, atomic absorption spectroscopy, is similar to wet chemistry in that the sample must still be dissolved, but it is much faster and cheaper. The solution is vaporized and its absorption spectrum is measured in the visible and ultraviolet range.[11]:225–226 Other techniques are X-ray fluorescence, electron microprobe analysis atom probe tomography and optical emission spectrography.[11]:227–232
Види још
Референце
- ^ „Mineralogy”. American Heritage Dictionary. Houghton Mifflin Harcourt Publishing Company. 2017. Приступљено 19. 10. 2017.
- ^ „Mineralogy”. Collins English Dictionary. HarperCollins Publishers. Приступљено 19. 10. 2017.
- ^ „NASA Instrument Inaugurates 3-D Moon Imaging”. JPL. Приступљено 19. 12. 2008.
- ^ Needham, Volume 3, 637.
- ^ Needham, Joseph (1959). Science and civilisation in China
 . Cambridge: Cambridge University Press. стр. 637–638. ISBN 978-0521058018.
. Cambridge: Cambridge University Press. стр. 637–638. ISBN 978-0521058018.
- ^ а б Needham, Volume 3, 636.
- ^ а б в г д ђ е Nesse, William D. (2012). Introduction to mineralogy (2nd изд.). New York: Oxford University Press. ISBN 978-0199827381.
- ^ „Law of the constancy of interfacial angles”. Online dictionary of crystallography. International Union of Crystallography. 24. 8. 2014. Приступљено 22. 9. 2015.
- ^ а б в Rafferty, John P. (2012). Geological sciences (1st изд.). New York: Britannica Educational Pub. in association with Rosen Educational Services. стр. 14–15. ISBN 9781615304950.
- ^ а б в г д ђ Klein, Cornelis; Philpotts, Anthony R. (2013). Earth materials : introduction to mineralogy and petrology. New York: Cambridge University Press. ISBN 9780521145213.
- ^ а б в г д ђ е Klein, Cornelis; Hurlbut, Jr., Cornelius S. (1993). Manual of mineralogy : (after James D. Dana) (21st изд.). New York: Wiley. ISBN 047157452X.
- ^ Sharp, T. (27. 11. 2014). „Bridgmanite – named at last”. Science. 346 (6213): 1057—1058. PMID 25430755. S2CID 206563252. doi:10.1126/science.1261887.
- ^ а б Ashcroft, Neil W.; Mermin, N. David (1977). Solid state physics
 (27. repr. изд.). New York: Holt, Rinehart and Winston. ISBN 9780030839931.
(27. repr. изд.). New York: Holt, Rinehart and Winston. ISBN 9780030839931.
- ^ Dinnebier, Robert E.; Billinge, Simon J.L. (2008). „1. Principles of powder diffraction”. Ур.: Dinnebier, Robert E.; Billinge, Simon J.L. Powder diffraction : theory and practice
 (Repr. изд.). Cambridge: Royal Society of Chemistry. стр. 1–19. ISBN 9780854042319.
(Repr. изд.). Cambridge: Royal Society of Chemistry. стр. 1–19. ISBN 9780854042319.
Литература
- Gribble, C.D.; Hall, A.J. (1993). Optical Mineralogy: Principles And Practice. London: CRC Press. ISBN 9780203498705.
- Harrell, James A. (2012). „Mineralogy”. Ур.: Bagnall, Roger S.; Brodersen, Kai; Champion, Craige B.; Erskine, Andrew. The encyclopedia of ancient history. Malden, MA: Wiley-Blackwell. ISBN 9781444338386. doi:10.1002/9781444338386.wbeah21217.
- Hazen, Robert M. (1984). „Mineralogy: A historical review” (PDF). Journal of Geological Education. 32 (5): 288–298. doi:10.5408/0022-1368-32.5.288. Приступљено 27. 9. 2017.
- Laudan, Rachel (1993). From mineralogy to geology : the foundations of a science, 1650-1830 (Pbk. изд.). Chicago: University of Chicago Press. ISBN 9780226469478.
- Oldroyd, David (1998). Sciences of the earth : studies in the history of mineralogy and geology. Aldershot: Ashgate. ISBN 9780860787709.
- Perkins, Dexter (2014). Mineralogy. Pearson Higher Ed. ISBN 9780321986573.
- Rapp, George R. (2002). Archaeomineralogy. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN 9783662050057.
- Tisljar, S.K. Haldar, Josip (2013). Introduction to mineralogy and petrology. Burlington: Elsevier Science. ISBN 9780124167100.
- Wenk, Hans-Rudolf; Bulakh, Andrey (2016). Minerals: Their Constitution and Origin. Cambridge University Press. ISBN 9781316425282.
- Whewell, William (2010). „Book XV. History of Mineralogy”. History of the Inductive Sciences: From the Earliest to the Present Times. Cambridge University Press. стр. 187–252. ISBN 9781108019262.
- Bandy, Mark Chance and Jean A. Bandy (1955). De Natura Fossilium. New York: George Banta Publishing Company.
- Chan, Alan Kam-leung and Gregory K. Clancey, Hui-Chieh Loy (2002). Historical Perspectives on East Asian Science, Technology and Medicine. Singapore: Singapore University Press ISBN 9971-69-259-7
- Hazen, Robert M. (1984). "Mineralogy: A historical review". Journal of Geological Education, 32, 288–298.
- Needham, Joseph (1986). Science and Civilization in China: Volume 3. Taipei: Caves Books, Ltd.
- Povarennykh A.S. (1972) "A Short History of Mineralogy and the Classification of Minerals". Crystal Chemical Classification of Minerals, 3–26. Springer, Boston, MA. ISBN 978-1-4684-1743-2
- Ramsdell, Lewis S. (1963). Encyclopedia Americana: International Edition: Volume 19. New York: Americana Corporation.
- Sivin, Nathan (1995). Science in Ancient China. Brookfield, Vermont: VARIORUM, Ashgate Publishing.
Спољашње везе
- The Virtual Museum of the History of Mineralogy
- Virtual Museum of the History of Mineralogy
- Georg Agricola's "Textbook on Mineralogy" on gemstones and minerals
Асоцијације
- American Federation of Mineral Societies
- French Society of Mineralogy and Crystallography
- Geological Society of America
- German Mineralogical Society
- International Mineralogical Association
- Italian Mineralogical and Petrological Society
- Mineralogical Association of Canada
- Mineralogical Society of Great Britain and Ireland
- Mineralogical Society of America

