Dabigatran
Appearance
 | |
 | |
| Klinički podaci | |
|---|---|
| Prodajno ime | Pradax, Pradaxa, Rendix |
| Drugs.com | Monografija |
| Način primene | Oralno |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 12-14 h |
| Izlučivanje | Urin (85%) |
| Identifikatori | |
| CAS broj | 211915-06-9 |
| ATC kod | B01AE07 (WHO) |
| PubChem | CID 6445226 |
| DrugBank | DB06695 |
| ChemSpider | 4948999 |
| ChEBI | CHEBI:70746 |
| ChEMBL | CHEMBL539697 |
| Hemijski podaci | |
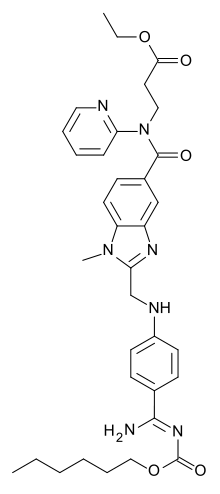
| Formula | C34H41N7O5 |
| Molarna masa | 627,733 |
| |
| |
| Fizički podaci | |
| Tačka topljenja | 180 °C (356 °F) |
Dabigatran je organsko jedinjenje, koje sadrži 34 atoma ugljenika i ima molekulsku masu od 627,733 Da.[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15]
Osobine
[uredi | uredi izvor]| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 10 |
| Broj donora vodonika | 2 |
| Broj rotacionih veza | 17 |
| Particioni koeficijent[16] (ALogP) | 5,8 |
| Rastvorljivost[17] (logS, log(mol/L)) | -9,2 |
| Polarna površina[18] (PSA, Å2) | 154,0 |
Reference
[uredi | uredi izvor]- ^ Bauer KA: New oral anticoagulants in development: potential for improved safety profiles. Rev Neurol Dis. 2010;7(1):1-8. PMID 20410856
- ^ Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L: Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51. Epub 2009 Aug 30. PMID 19717844
- ^ Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI: Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost. 2009 Jan;101(1):77-85. PMID 19132192
- ^ Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA: Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009 Jan;24(1):1-9. Epub 2008 Apr 14. PMID 18534438
- ^ Eriksson BI, Dahl OE, Buller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kalebo P, Reilly P: A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005 Jan;3(1):103-11. PMID 15634273
- ^ Di Nisio M, Middeldorp S, Buller HR: Direct thrombin inhibitors. N Engl J Med. 2005 Sep 8;353(10):1028-40. PMID 16148288
- ^ Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, Rathgen K, Svard R: Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005 May;45(5):555-63. PMID 15831779
- ^ Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, Pedersen KE, Lionetti DA, Stangier J, Wallentin L: Dabigatran with or without concomitant aspirin compared with warfarinalone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007 Nov 1;100(9):1419-26. Epub 2007 Aug 17. PMID 17950801
- ^ Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kalebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Buller HR: Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007 Nov;5(11):2178-85. PMID 17764540
- ^ Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR: Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007 Sep 15;370(9591):949-56. PMID 17869635
- ^ European Medicines Agency:http://www.ema.europa.eu/humandocs/PDFs/EPAR/pradaxa/H-829-en6.pdf[мртва веза]
- ^ Abrams P and Marzella N: Dabigatran (Rendix): A Promising New Oral Direct Thrombin Inhibitor. Drug Forecast. 2007;32(5):271-5. pharmscope:http://www.pharmscope.com/ptjournal/fulltext/32/5/PTJ3205271.pdf Архивирано на сајту Wayback Machine (4. март 2016)
- ^ Scaglione F: New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013 Feb;52(2):69-82. doi: 10.1007/s40262-012-0030-9. PMID 23292752
- ^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMC 3013709
 . PMID 21059682. doi:10.1093/nar/gkq1126.
. PMID 21059682. doi:10.1093/nar/gkq1126.
- ^ David S. Wishart; Craig Knox; An Chi Guo; Dean Cheng; Savita Shrivastava; Dan Tzur; Bijaya Gautam; Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic acids research. 36 (Database issue): D901—6. PMC 2238889
 . PMID 18048412. doi:10.1093/nar/gkm958.
. PMID 18048412. doi:10.1093/nar/gkm958.
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura
[uredi | uredi izvor]- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.
Spoljašnje veze
[uredi | uredi izvor]
 | Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |
