Supstitucija (hemija) — разлика између измена
Add 1 book for Википедија:Проверљивост (20220205sim)) #IABot (v2.0.8.6) (GreenC bot |
. |
||
| Ред 1: | Ред 1: | ||
{{short description|Hemijska reakcija u kojoj se jedna funkcionalna grupa u jedinjenju zamenjuje drugom}}{{rut}} |
|||
| ⚫ | U '''supstitucionim reakcijama''', [[funkcionalna grupa]] jednog [[hemijsko jedinjenje|hemijskog jedinjenja]] se zamenjuje drugom grupom.<ref>{{ |
||
| ⚫ | U '''supstitucionim reakcijama''', [[funkcionalna grupa]] jednog [[hemijsko jedinjenje|hemijskog jedinjenja]] se zamenjuje drugom grupom.<ref name=":0">{{JerryMarch}}</ref><ref name=":1">{{cite journal |title=Is This Reaction a Substitution, Oxidation-Reduction, or Transfer? |first=Naum S. |last=Imyanitov |journal=J. Chem. Educ. |year=1993 |volume=70 |issue=1 |pages=14–16 |doi=10.1021/ed070p14 |bibcode = 1993JChEd..70...14I }}</ref> U [[organska hemija|organskoj hemiji]], reakcije [[elektrofil]]ne i [[nukleofil]]ne supstitucije imaju primarni značaj. Reakcije organske supstitucije se klasifikuju u nekoliko glavnih tipova [[Organska sinteza|organskih reakcija]] u zavisnosti od toga da li je [[reagens]] elektrofil ili nukleofil, da li je reakcioni intermedijar [[karbokatjon]], [[karbanjon]] ili [[Radikal (hemija)|slobodni radikal]], ili da li je [[enzimski supstrat (biohemija)|supstrat]] [[Alifatično jedinjenje|alifatičan]] ili [[aromatičnost|aromatičan]]. Detaljno razumevanje tipa reakcije pomaže u predviđanju produkta reakcije. To je takođe korisno za optimizaciju reakcije u pogledu promenljivih, kao što su temperatura i izvor [[rastvarač]]a. |
||
Dobar primer reakcije supstitucije je [[fotohemija|fotohemijska]] [[hlorinacija]] [[metan]]a kojom se formira [[hlorometan|metil hlorid]]. |
|||
Dobar primer reakcije supstitucije je [[fotohemija|fotohemijska]] [[hlorinacija]] [[metan]]a kojom se formira [[hlorometan|metil hlorid]]. When [[chlorine]] gas (Cl<sub>2</sub>) is irradiated, some of the molecules are split into two chlorine [[radical (chemistry)|radicals]] (Cl•), whose free electrons are strongly [[nucleophilic]]. One of them breaks a [[carbon–hydrogen bond|C–H covalent bond]] in CH<sub>4</sub> and grabs the hydrogen atom to form the electrically neutral HCl. The other radical reforms a covalent bond with the CH<sub>3</sub>• to form CH<sub>3</sub>Cl ([[methyl chloride]]). |
|||
{| class="wikitable" |
{| class="wikitable" |
||
| Ред 12: | Ред 14: | ||
== Nukleofilna supstitucija == |
== Nukleofilna supstitucija == |
||
{{Main-lat|Nukleofilna supstitucija}} |
|||
Nukleofilna supstitucija se odvija kad je reagens [[nukleofil]] (atom ili molekul sa slobodnim elektronima). |
|||
Nukleofil reaguje sa [[Alifatično jedinjenje|alifatičnim]] supstratom u reakciji [[Nukleofilna supstitucija|nukleofilne alifatične supstitucija]]. Ta supstitucija se može odvijati putem dva različita mehanizma: unimolekularna nukleofilna supstitucija (-{SN}-<sub>1</sub>) i bimolekularna nukleofilna supstitucija (-{SN}-<sub>2</sub>). -{SN}-<sub>1</sub> mehanizam ima dva stupnja. U prvom stupnju, odlazeća grupa se udalji, čime se formira karbokatjon. U drugom stupnju, nukleofilni reagens napada karbokatjon i formira sigma vezu. Ovaj mehanizam može da proizvede bilo inverziju ili zadržavanje konfiguracije. -{SN}-<sub>2</sub> reakcija ima samo jedan stupanj. Napad reagensa i uklanjanje odlazeće grupe se odvijaju istovremeno. Ovaj mehanizam uvek dovodi do inverzije konfiguracije.<ref name="Clayden1st">{{Clayden1st}}</ref> |
Nukleofilna supstitucija se odvija kad je reagens [[nukleofil]] (atom ili molekul sa slobodnim elektronima).<ref name=":0" /><ref name=":1" /> Nukleofil reaguje sa [[Alifatično jedinjenje|alifatičnim]] supstratom u reakciji [[Nukleofilna supstitucija|nukleofilne alifatične supstitucija]].<ref>{{cite journal | vauthors = Neumann CN, Hooker JM, Ritter T | title = Concerted nucleophilic aromatic substitution with (19)F(-) and (18)F(-) | journal = Nature | volume = 534 | issue = 7607 | pages = 369–73 | date = June 2016 | pmid = 27281221 | pmc = 4911285 | doi = 10.1038/nature17667 }}</ref><ref>{{cite journal | vauthors = Rohrbach S, Smith AJ, Pang JH, Poole DL, Tuttle T, Chiba S, Murphy JA | title = Concerted Nucleophilic Aromatic Substitution Reactions | journal = Angewandte Chemie | volume = 58 | issue = 46 | pages = 16368–16388 | date = November 2019 | pmid = 30990931 | pmc = 6899550 | doi = 10.1002/anie.201902216 }}</ref> Ta supstitucija se može odvijati putem dva različita mehanizma: unimolekularna nukleofilna supstitucija (-{SN}-<sub>1</sub>) i bimolekularna nukleofilna supstitucija (-{SN}-<sub>2</sub>). -{SN}-<sub>1</sub> mehanizam ima dva stupnja. U prvom stupnju, odlazeća grupa se udalji, čime se formira karbokatjon. U drugom stupnju, nukleofilni reagens napada karbokatjon i formira sigma vezu. Ovaj mehanizam može da proizvede bilo inverziju ili zadržavanje konfiguracije. -{SN}-<sub>2</sub> reakcija ima samo jedan stupanj. Napad reagensa i uklanjanje odlazeće grupe se odvijaju istovremeno. Ovaj mehanizam uvek dovodi do inverzije konfiguracije.<ref name="Clayden1st">{{Clayden1st}}</ref> |
||
Kad je supstrat [[aromatičnost|aromatično]] jedinjenje reakcioni tip je [[nukleofilna aromatična supstitucija]]. Derivati [[karboksilna kiselina|karboksilnih kiselina]] reaguju sa nukleofilima u [[nukleofilna acilna supstitucija|nukleofilnoj acilnoj supstituciji]]. |
Kad je supstrat [[aromatičnost|aromatično]] jedinjenje reakcioni tip je [[nukleofilna aromatična supstitucija]]. Derivati [[karboksilna kiselina|karboksilnih kiselina]] reaguju sa nukleofilima u [[nukleofilna acilna supstitucija|nukleofilnoj acilnoj supstituciji]].<ref name=goldstein>{{cite journal | vauthors = Goldstein SW, Bill A, Dhuguru J, Ghoneim O | title = Nucleophilic Aromatic Substitution Addition and Identification of an Amine. | journal = Journal of Chemical Education | date = September 2017 | volume = 94 | issue = 9 | pages = 1388–90 | doi = 10.1021/acs.jchemed.6b00680 | bibcode = 2017JChEd..94.1388G }}</ref> |
||
The most general form for the reaction may be given as |
|||
: Nuc''':''' + R-LG → R-Nuc + LG''':''' |
|||
where R-LG indicates the substrate. The electron pair (''':''') from the nucleophile (Nuc:) attacks the substrate (R-LG), forming a new covalent bond Nuc-R-LG. The prior state of charge is restored when the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. In such reactions, the nucleophile is usually electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. |
|||
An example of nucleophilic substitution is the hydrolysis of an [[alkyl]] bromide, R-Br, under basic conditions, where the ''attacking'' nucleophile is the base OH<sup>−</sup> and the leaving group is Br<sup>−</sup>: |
|||
: R-Br + OH<sup>−</sup> → R-OH + Br<sup>−</sup> |
|||
Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorized as taking place at a carbon of a saturated aliphatic compound carbon or (less often) at an aromatic or other unsaturated carbon center.<ref name=":0" /> |
|||
=== Mehanizmi === |
|||
{{Main-lat|SN1 reaction|SN2 reaction|Nucleophilic aromatic substitution}} |
|||
Nucleophilic substitutions on aliphatic carbon centers can proceed by two different mechanisms, unimolecular nucleophilic substitution ([[SN1 reaction|S<sub>N</sub>1]]) and bimolecular nucleophilic substitution ([[SN2 reaction|S<sub>N</sub>2]]). |
|||
The S<sub>N</sub>1 mechanism has two steps. In the first step, the leaving group departs, forming a [[carbocation]] C<sup>+</sup>. In the second step, the nucleophilic reagent (Nuc:) attaches to the carbocation and forms a covalent sigma bond. If the substrate has a [[Chirality|chiral]] carbon, this mechanism can result in either inversion of the [[stereochemistry]] or retention of configuration. Usually, both occur without preference. The result is [[racemization]]. |
|||
The S<sub>N</sub>2 mechanism has just one step. The attack of the reagent and the expulsion of the leaving group happen simultaneously. This mechanism always results in inversion of configuration. If the substrate that is under nucleophilic attack is chiral, the reaction will therefore lead to an inversion of its [[stereochemistry]], called a [[Walden inversion]]. |
|||
S<sub>N</sub>2 attack may occur if the backside route of attack is not [[sterically hindered]] by substituents on the substrate. Therefore, this mechanism usually occurs at an unhindered primary carbon center. If there is steric crowding on the substrate near the leaving group, such as at a tertiary carbon center, the substitution will involve an S<sub>N</sub>1 rather than an S<sub>N</sub>2 mechanism; an S<sub>N</sub>1 would also be more likely in this case because a sufficiently stable carbocation intermediary could be formed. |
|||
When the substrate is an [[aromatic]] compound, the reaction type is [[nucleophilic aromatic substitution]], which occur with various mechanisms. [[Carboxylic acid]] derivatives react with nucleophiles in [[nucleophilic acyl substitution]]. This kind of reaction can be useful in preparing compounds. |
|||
== Elektrofilna supstitucija == |
== Elektrofilna supstitucija == |
||
{{Main-lat|Elektrofilna supstitucija}} |
|||
[[Elektrofil]]i učestvuju u reakcijama [[elektrofilna supstitucija|elektrofilne supstitucije]], a posebno u [[elektrofilna aromatična supstitucija|elektrofilnim aromatičnim supstitucijama]]. |
|||
[[Elektrofil]]i učestvuju u reakcijama [[elektrofilna supstitucija|elektrofilne supstitucije]], a posebno u [[elektrofilna aromatična supstitucija|elektrofilnim aromatičnim supstitucijama]].<ref>{{March6th}}</ref><ref>{{Cite journal|last=Gawley|first=Robert E.|date=1999-06-04|title=A proposal for (slight) modification of the Hughes–Ingold mechanistic descriptors for substitution reactions|journal=Tetrahedron Letters|volume=40|issue=23|pages=4297–4300|doi=10.1016/S0040-4039(99)00780-7|issn=0040-4039}}</ref> |
|||
{| class="wikitable" |
{| class="wikitable" |
||
| Ред 30: | Ред 55: | ||
Elektrofilne reakcije sa drugim nezasićenim jedinjenjima, osim [[aromatični ugljovodonici|arena]], generalno dovode do [[elektrofilna adicija|elektrofilne adicije]] umesto supstitucije. |
Elektrofilne reakcije sa drugim nezasićenim jedinjenjima, osim [[aromatični ugljovodonici|arena]], generalno dovode do [[elektrofilna adicija|elektrofilne adicije]] umesto supstitucije. |
||
== Radical substitution == |
|||
A [[radical substitution]] reaction involves [[radical (chemistry)|radicals]].<ref name=":0" /> An example is the [[Hunsdiecker reaction]].<ref name = JJL /><ref name = Borodin1861a /><ref name = Borodin1861b /><ref name = Simonini1892 /><ref name = Simonini1893 /> |
|||
==Organometallic substitution== |
|||
[[Coupling reaction]]s are a class of metal-catalyzed reactions involving an [[Organometallics|organometallic]] compound RM and an organic halide R′X that together react to form a compound of the type R-R′ with formation of a new [[carbon–carbon bond]]. Examples include the [[Heck reaction]], [[Ullmann reaction]], and [[Wurtz–Fittig reaction]]. Many variations exist.<ref>{{cite book |last=Elschenbroich |first=C. |last2=Salzer |first2=A. |title=Organometallics: A Concise Introduction |edition=2nd |year=1992 |publisher=Wiley-VCH |location=Weinheim |isbn=3-527-28165-7 }}</ref> |
|||
== Substituted compounds == |
|||
'''Substituted compounds''' are [[chemical compound]]s where one or more [[hydrogen]] [[atom]]s of a core structure have been replaced with a [[functional group]] like [[alkyl]], [[Hydroxyl|hydroxy]], or [[halogen]], or with larger [[substituent]] groups. |
|||
For example, [[benzene]] is a [[simple aromatic ring]]. Benzenes that have undergone substitution are a [[heterogeneous]] group of chemicals with a wide spectrum of uses and properties: |
|||
{|align="center" class="wikitable" |
|||
| colspan=3 style="background: #ccccff; text-align: center"| '''Examples of substituted benzene compounds''' |
|||
|- |
|||
|'''compound'''||'''general formula'''||'''general structure''' |
|||
|- |
|||
|[[Benzene]] ||C<sub>6</sub>H<sub>6</sub>||<center>[[Image:Benz4.png|70px]]</center> |
|||
|- |
|||
|[[Toluene]] ||C<sub>6</sub>H<sub>5</sub>-CH<sub>3</sub>||<center>[[Image:Toluol.svg|40px]]</center> |
|||
|- |
|||
|[[o-Xylene]] || C<sub>6</sub>H<sub>4</sub>(-CH<sub>3</sub>)<sub>2</sub>||<center>[[Image:O-xylene.png|60px]]</center> |
|||
|- |
|||
|[[Mesitylene]] ||C<sub>6</sub>H<sub>3</sub>(-CH<sub>3</sub>)<sub>3</sub>||<center>[[Image:1,3,5-Trimethylbenzene.svg|70px]]</center> |
|||
|- |
|||
|[[Phenol]] ||C<sub>6</sub>H<sub>5</sub>-OH||<center>[[Image:Phenol-2D-skeletal.png|50px]]</center> |
|||
|} |
|||
== Vidi još == |
== Vidi još == |
||
| Ред 36: | Ред 87: | ||
== Reference == |
== Reference == |
||
{{reflist| |
{{reflist|refs= |
||
<ref name = JJL>{{cite book|title = Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications|edition = 5th|publisher = [[Springer Science & Business Media]]|pages = 327–328|first = J. J.|last = Li|chapter = Hunsdiecker–Borodin Reaction|chapter-url = https://books.google.com/books?id=HoXBBAAAQBAJ&pg=PA327|isbn = 9783319039794|date = 2014-01-30}}</ref> |
|||
<ref name = Borodin1861a>{{cite journal|first = A.|last = Borodin|authorlink = Alexander Borodin|journal = [[Annalen der Chemie und Pharmacie]]|year = 1861|volume = 119|pages = 121–123|doi = 10.1002/jlac.18611190113|title = Ueber Bromvaleriansäure und Brombuttersäure|language = German|trans-title = About bromovaleric acid and bromobutyric acid|url = https://zenodo.org/record/1427169}}</ref> |
|||
<ref name = Borodin1861b>{{cite journal|first = A.|last = Borodin|authorlink = Alexander Borodin|journal = [[Zeitschrift für Chemie und Pharmacie]]|year = 1861|volume = 4|pages = 5–7|url = https://babel.hathitrust.org/cgi/pt?id=uc1.b3645096;view=1up;seq=39|title = Ueber de Monobrombaldriansäure und Monobrombuttersäure|language = German|trans-title = About the monobromovaleric acid and monobromobutyric acid}}</ref> |
|||
<ref name = Simonini1892>{{cite journal|title = Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen|language = German|trans-title = About the breakdown of fatty acids to lower carbon alcohols|last = Simonini|first = A.|journal = [[Monatshefte für Chemie und verwandte Teile anderer Wissenschaften]]|year = 1892|volume = 13|issue = 1|pages = 320–325|doi = 10.1007/BF01523646|s2cid = 197766447}}</ref> |
|||
<ref name = Simonini1893>{{cite journal|title = Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen|language = German|trans-title = About the breakdown of fatty acids to lower carbon alcohols|last = Simonini|first = A.|journal = [[Monatshefte für Chemie und verwandte Teile anderer Wissenschaften]]|year = 1893|volume = 14|issue = 1|pages = 81–92|doi = 10.1007/BF01517859|s2cid = 104367588}}</ref> |
|||
}} |
|||
== Literatura == |
== Literatura == |
||
{{refbegin|30em}} |
|||
* R. A. Rossi, R. H. de Rossi, ''Aromatic Substitution by the S<sub>RN</sub>1 Mechanism, ACS Monograph Series No. 178, American Chemical Society, 1983. {{ISBN|0-8412-0648-1}} |
|||
* L. G. Wade, ''Organic Chemistry'', 5th ed., Prentice Hall, Upper Saddle River, New Jersey, 2003. |
|||
* S. R. Hartshorn, ''Aliphatic Nucleophilic Substitution'', Cambridge University Press, London, 1973. {{ISBN|0-521-09801-7}} |
|||
* ''Introducing Aliphatic Substitution with a Discovery Experiment Using Competing Electrophiles'' Timothy P. Curran, Amelia J. Mostovoy, Margaret E. Curran, and Clara Berger Journal of Chemical Education 2016 93 (4), 757-761 {{DOI|10.1021/acs.jchemed.5b00394}} |
|||
* N.S.Imyanitov. ''Electrophilic Bimolecular Substitution as an Alternative to Nucleophilic Monomolecular Substitution in Inorganic and Organic Chemistry''. J. Gen. Chem. USSR (Engl. Transl.) '''1990'''; 60 (3); 417-419. |
|||
* Unimolecular Nucleophilic Substitution does not Exist / N.S.Imyanitov. [http://sciteclibrary.ru/eng/catalog/pages/9330.html SciTecLibrary] |
|||
* {{cite journal |last1=Lenoir |first1=D. |last2=Chiappe |first2=C. |title=What is the Nature of the First-Formed Intermediates in the Electrophilic Halogenation of Alkenes, Alkynes, and Allenes? |journal=[[Chemistry: A European Journal|Chem. Eur. J.]] |year=2003 |volume=9 |issue=5 |pages=1036–1044 |doi=10.1002/chem.200390097 |pmid=12596140 }} |
|||
* {{cite journal |last=Brown |first=R. S. |title=Investigation of the Early Steps in Electrophilic Bromination through the Study of the Reaction with Sterically Encumbered Olefins |journal=[[Accounts of Chemical Research|Acc. Chem. Res.]] |year=1997 |volume=30 |issue=3 |pages=131–137 |doi=10.1021/ar960088e }} |
|||
* {{Cite book |last1=Vollhardt |first1=K. Peter C. |last2=Schore |first2=Neil Eric |url=https://www.worldcat.org/oclc/866584251|title=Organic chemistry : structure and function|date=January 2014 |isbn=978-1-4641-2027-5|edition=7th|location=New York, NY|oclc=866584251}} |
|||
* {{Cite journal|last1=Fahey|first1=Robert C.|last2=Lee|first2=Do-Jae.|date=April 1968|title=Polar additions to olefins and acetylenes. V. Bimolecular and termolecular mechanisms in the hydrochlorination of acetylenes|journal=Journal of the American Chemical Society|language=EN|volume=90|issue=8|pages=2124–2131|doi=10.1021/ja01010a034|issn=0002-7863}} |
|||
* {{Cite book|title=Perspectives on structure and mechanism in organic chemistry|last=A.|first=Carroll, Felix|date=2010|publisher=John Wiley|isbn=9780470276105|edition=2nd|location=Hoboken, N.J.|oclc=286483846}} |
|||
* {{Cite journal|last1=Mootz|first1=Dietrich|last2=Deeg|first2=Axel|date=July 1992|title=2-Butyne and hydrogen chloride cocrystallized: solid-state geometry of Cl-H.cntdot..cntdot..cntdot..pi. hydrogen bonding to the carbon-carbon triple bond|journal=Journal of the American Chemical Society|language=EN|volume=114|issue=14|pages=5887–5888|doi=10.1021/ja00040a077|issn=0002-7863}} |
|||
* {{Cite book|title=Mechanism and theory in organic chemistry|last=H.|first=Lowry, Thomas|date=1987|publisher=Harper & Row|others=Richardson, Kathleen Schueller.|isbn=978-0060440848|edition=3rd|location=New York|oclc=14214254|url-access=registration|url=https://archive.org/details/mechanismtheoryi000321}} |
|||
* {{cite journal |last1=Wang |first1=Z. |last2=Tu |first2=Y. |last3=Frohn |first3=M. |last4=Zhang |first4=J. |last5=Shi |first5=Y. |title=An Efficient Catalytic Asymmetric Epoxidation Method |journal=[[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |year=1997 |volume=119 |issue=46 |pages=11224–11235 |doi=10.1021/ja972272g }} |
|||
* {{cite journal |last1=Davis |first1=F. A. |last2=Kumar |first2=A. |last3=Chen |first3=B. C. |title=Chemistry of oxaziridines. 16. A short, highly enantioselective synthesis of the AB-ring segments of γ-rhodomycionone and α-citromycinone using (+)-[(8,8-dimethoxycamphoryl)sulfonyl]oxaziridine |journal=[[Journal of Organic Chemistry|J. Org. Chem.]] |year=1991 |volume=56 |issue=3 |pages=1143–1145 |doi= 10.1021/jo00003a042 }} |
|||
* {{cite journal |last1=Uehlin |first1=L. |last2=Wirth |first2=T. |title=Novel Polymer-Bound Chiral Selenium Electrophiles |journal=Org. Lett. |year=2001 |volume=3 |issue=18 |pages=2931–2933 |doi=10.1021/ol0164435 |pmid=11529793 }} |
|||
* {{cite journal | vauthors = Tjosaas F, Fiksdahl A | title = A simple synthetic route to methyl 3-fluoropyridine-4-carboxylate by nucleophilic aromatic substitution | journal = Molecules (Basel, Switzerland) | volume = 11 | issue = 2 | pages = 130–3 | date = February 2006 | pmid = 17962783 | pmc = 6148553 | doi = 10.3390/11020130 | doi-access = free }} |
|||
* {{cite journal | vauthors = Bella M, Kobbelgaard S, Jørgensen KA | title = Organocatalytic regio- and asymmetric C-selective S(N)Ar reactions-stereoselective synthesis of optically active spiro-pyrrolidone-3,3'-oxoindoles | journal = Journal of the American Chemical Society | volume = 127 | issue = 11 | pages = 3670–1 | date = March 2005 | pmid = 15771481 | doi = 10.1021/ja050200g }} |
|||
{{refend}} |
|||
== Спољашње везе == |
== Спољашње везе == |
||
{{Commonscat|Substitution reactions}} |
{{Commonscat-lat|Substitution reactions}} |
||
* {{Cite web|title=Nucleophiles and Electrophiles|url=http://butane.chem.uiuc.edu/pshapley/genchem2/b5/1.html|access-date=2020-09-21|website=butane.chem.uiuc.edu}} |
|||
* {{Cite web|title=Electrophile {{!}} chemistry|url=https://www.britannica.com/science/electrophile|access-date=2020-09-21|website=Encyclopedia Britannica|language=en}} |
|||
* [https://archive.today/20121215085720/http://www.cem.msu.edu/~reusch/VirtTxtJml/benzrx1.htm Aromatic Substitution Reactions – MSU] |
|||
{{-}} |
|||
{{Organske reakcije-lat}} |
{{Organske reakcije-lat}} |
||
{{Authority control-lat}} |
|||
{{нормативна контрола-лат}} |
|||
[[Категорија:Хемијске реакције]] |
[[Категорија:Хемијске реакције]] |
||
Верзија на датум 8. јул 2022. у 23:05
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
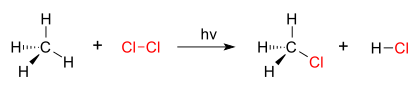
U supstitucionim reakcijama, funkcionalna grupa jednog hemijskog jedinjenja se zamenjuje drugom grupom.[1][2] U organskoj hemiji, reakcije elektrofilne i nukleofilne supstitucije imaju primarni značaj. Reakcije organske supstitucije se klasifikuju u nekoliko glavnih tipova organskih reakcija u zavisnosti od toga da li je reagens elektrofil ili nukleofil, da li je reakcioni intermedijar karbokatjon, karbanjon ili slobodni radikal, ili da li je supstrat alifatičan ili aromatičan. Detaljno razumevanje tipa reakcije pomaže u predviđanju produkta reakcije. To je takođe korisno za optimizaciju reakcije u pogledu promenljivih, kao što su temperatura i izvor rastvarača.
Dobar primer reakcije supstitucije je fotohemijska hlorinacija metana kojom se formira metil hlorid. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons are strongly nucleophilic. One of them breaks a C–H covalent bond in CH4 and grabs the hydrogen atom to form the electrically neutral HCl. The other radical reforms a covalent bond with the CH3• to form CH3Cl (methyl chloride).
| hlorinacija metana hlorom |
|---|
Nukleofilna supstitucija
Nukleofilna supstitucija se odvija kad je reagens nukleofil (atom ili molekul sa slobodnim elektronima).[1][2] Nukleofil reaguje sa alifatičnim supstratom u reakciji nukleofilne alifatične supstitucija.[3][4] Ta supstitucija se može odvijati putem dva različita mehanizma: unimolekularna nukleofilna supstitucija (SN1) i bimolekularna nukleofilna supstitucija (SN2). SN1 mehanizam ima dva stupnja. U prvom stupnju, odlazeća grupa se udalji, čime se formira karbokatjon. U drugom stupnju, nukleofilni reagens napada karbokatjon i formira sigma vezu. Ovaj mehanizam može da proizvede bilo inverziju ili zadržavanje konfiguracije. SN2 reakcija ima samo jedan stupanj. Napad reagensa i uklanjanje odlazeće grupe se odvijaju istovremeno. Ovaj mehanizam uvek dovodi do inverzije konfiguracije.[5]
Kad je supstrat aromatično jedinjenje reakcioni tip je nukleofilna aromatična supstitucija. Derivati karboksilnih kiselina reaguju sa nukleofilima u nukleofilnoj acilnoj supstituciji.[6]
The most general form for the reaction may be given as
- Nuc: + R-LG → R-Nuc + LG:
where R-LG indicates the substrate. The electron pair (:) from the nucleophile (Nuc:) attacks the substrate (R-LG), forming a new covalent bond Nuc-R-LG. The prior state of charge is restored when the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. In such reactions, the nucleophile is usually electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged.
An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br, under basic conditions, where the attacking nucleophile is the base OH− and the leaving group is Br−:
- R-Br + OH− → R-OH + Br−
Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorized as taking place at a carbon of a saturated aliphatic compound carbon or (less often) at an aromatic or other unsaturated carbon center.[1]
Mehanizmi
Nucleophilic substitutions on aliphatic carbon centers can proceed by two different mechanisms, unimolecular nucleophilic substitution (SN1) and bimolecular nucleophilic substitution (SN2).
The SN1 mechanism has two steps. In the first step, the leaving group departs, forming a carbocation C+. In the second step, the nucleophilic reagent (Nuc:) attaches to the carbocation and forms a covalent sigma bond. If the substrate has a chiral carbon, this mechanism can result in either inversion of the stereochemistry or retention of configuration. Usually, both occur without preference. The result is racemization.
The SN2 mechanism has just one step. The attack of the reagent and the expulsion of the leaving group happen simultaneously. This mechanism always results in inversion of configuration. If the substrate that is under nucleophilic attack is chiral, the reaction will therefore lead to an inversion of its stereochemistry, called a Walden inversion.
SN2 attack may occur if the backside route of attack is not sterically hindered by substituents on the substrate. Therefore, this mechanism usually occurs at an unhindered primary carbon center. If there is steric crowding on the substrate near the leaving group, such as at a tertiary carbon center, the substitution will involve an SN1 rather than an SN2 mechanism; an SN1 would also be more likely in this case because a sufficiently stable carbocation intermediary could be formed.
When the substrate is an aromatic compound, the reaction type is nucleophilic aromatic substitution, which occur with various mechanisms. Carboxylic acid derivatives react with nucleophiles in nucleophilic acyl substitution. This kind of reaction can be useful in preparing compounds.
Elektrofilna supstitucija
Elektrofili učestvuju u reakcijama elektrofilne supstitucije, a posebno u elektrofilnim aromatičnim supstitucijama.[7][8]
| Elektrofilna aromatična supstitucija |
|---|
Elektrofilne reakcije sa drugim nezasićenim jedinjenjima, osim arena, generalno dovode do elektrofilne adicije umesto supstitucije.
Radical substitution
A radical substitution reaction involves radicals.[1] An example is the Hunsdiecker reaction.[9][10][11][12][13]
Organometallic substitution
Coupling reactions are a class of metal-catalyzed reactions involving an organometallic compound RM and an organic halide R′X that together react to form a compound of the type R-R′ with formation of a new carbon–carbon bond. Examples include the Heck reaction, Ullmann reaction, and Wurtz–Fittig reaction. Many variations exist.[14]
Substituted compounds
Substituted compounds are chemical compounds where one or more hydrogen atoms of a core structure have been replaced with a functional group like alkyl, hydroxy, or halogen, or with larger substituent groups.
For example, benzene is a simple aromatic ring. Benzenes that have undergone substitution are a heterogeneous group of chemicals with a wide spectrum of uses and properties:
| Examples of substituted benzene compounds | ||
| compound | general formula | general structure |
| Benzene | C6H6 | 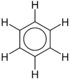 |
| Toluene | C6H5-CH3 | |
| o-Xylene | C6H4(-CH3)2 | 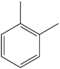 |
| Mesitylene | C6H3(-CH3)3 |  |
| Phenol | C6H5-OH | 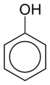 |
Vidi još
Reference
- ^ а б в г March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd изд.), New York: Wiley, ISBN 0-471-85472-7
- ^ а б Imyanitov, Naum S. (1993). „Is This Reaction a Substitution, Oxidation-Reduction, or Transfer?”. J. Chem. Educ. 70 (1): 14—16. Bibcode:1993JChEd..70...14I. doi:10.1021/ed070p14.
- ^ Neumann CN, Hooker JM, Ritter T (јун 2016). „Concerted nucleophilic aromatic substitution with (19)F(-) and (18)F(-)”. Nature. 534 (7607): 369—73. PMC 4911285
 . PMID 27281221. doi:10.1038/nature17667.
. PMID 27281221. doi:10.1038/nature17667.
- ^ Rohrbach S, Smith AJ, Pang JH, Poole DL, Tuttle T, Chiba S, Murphy JA (новембар 2019). „Concerted Nucleophilic Aromatic Substitution Reactions”. Angewandte Chemie. 58 (46): 16368—16388. PMC 6899550
 . PMID 30990931. doi:10.1002/anie.201902216.
. PMID 30990931. doi:10.1002/anie.201902216.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (I изд.). Oxford University Press. ISBN 978-0-19-850346-0.
- ^ Goldstein SW, Bill A, Dhuguru J, Ghoneim O (септембар 2017). „Nucleophilic Aromatic Substitution Addition and Identification of an Amine.”. Journal of Chemical Education. 94 (9): 1388—90. Bibcode:2017JChEd..94.1388G. doi:10.1021/acs.jchemed.6b00680.
- ^ Smith, Michael B.; March, Jerry (2007). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th изд.). New York: Wiley-Interscience. ISBN 0-471-72091-7.
- ^ Gawley, Robert E. (1999-06-04). „A proposal for (slight) modification of the Hughes–Ingold mechanistic descriptors for substitution reactions”. Tetrahedron Letters. 40 (23): 4297—4300. ISSN 0040-4039. doi:10.1016/S0040-4039(99)00780-7.
- ^ Li, J. J. (2014-01-30). „Hunsdiecker–Borodin Reaction”. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (5th изд.). Springer Science & Business Media. стр. 327—328. ISBN 9783319039794.
- ^ Borodin, A. (1861). „Ueber Bromvaleriansäure und Brombuttersäure” [About bromovaleric acid and bromobutyric acid]. Annalen der Chemie und Pharmacie (на језику: German). 119: 121—123. doi:10.1002/jlac.18611190113.
- ^ Borodin, A. (1861). „Ueber de Monobrombaldriansäure und Monobrombuttersäure” [About the monobromovaleric acid and monobromobutyric acid]. Zeitschrift für Chemie und Pharmacie (на језику: German). 4: 5—7.
- ^ Simonini, A. (1892). „Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen” [About the breakdown of fatty acids to lower carbon alcohols]. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften (на језику: German). 13 (1): 320—325. S2CID 197766447. doi:10.1007/BF01523646.
- ^ Simonini, A. (1893). „Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen” [About the breakdown of fatty acids to lower carbon alcohols]. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften (на језику: German). 14 (1): 81—92. S2CID 104367588. doi:10.1007/BF01517859.
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd изд.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
Literatura
- R. A. Rossi, R. H. de Rossi, Aromatic Substitution by the SRN1 Mechanism, ACS Monograph Series No. 178, American Chemical Society, 1983. ISBN 0-8412-0648-1
- L. G. Wade, Organic Chemistry, 5th ed., Prentice Hall, Upper Saddle River, New Jersey, 2003.
- S. R. Hartshorn, Aliphatic Nucleophilic Substitution, Cambridge University Press, London, 1973. ISBN 0-521-09801-7
- Introducing Aliphatic Substitution with a Discovery Experiment Using Competing Electrophiles Timothy P. Curran, Amelia J. Mostovoy, Margaret E. Curran, and Clara Berger Journal of Chemical Education 2016 93 (4), 757-761 doi:10.1021/acs.jchemed.5b00394
- N.S.Imyanitov. Electrophilic Bimolecular Substitution as an Alternative to Nucleophilic Monomolecular Substitution in Inorganic and Organic Chemistry. J. Gen. Chem. USSR (Engl. Transl.) 1990; 60 (3); 417-419.
- Unimolecular Nucleophilic Substitution does not Exist / N.S.Imyanitov. SciTecLibrary
- Lenoir, D.; Chiappe, C. (2003). „What is the Nature of the First-Formed Intermediates in the Electrophilic Halogenation of Alkenes, Alkynes, and Allenes?”. Chem. Eur. J. 9 (5): 1036—1044. PMID 12596140. doi:10.1002/chem.200390097.
- Brown, R. S. (1997). „Investigation of the Early Steps in Electrophilic Bromination through the Study of the Reaction with Sterically Encumbered Olefins”. Acc. Chem. Res. 30 (3): 131—137. doi:10.1021/ar960088e.
- Vollhardt, K. Peter C.; Schore, Neil Eric (јануар 2014). Organic chemistry : structure and function (7th изд.). New York, NY. ISBN 978-1-4641-2027-5. OCLC 866584251.
- Fahey, Robert C.; Lee, Do-Jae. (април 1968). „Polar additions to olefins and acetylenes. V. Bimolecular and termolecular mechanisms in the hydrochlorination of acetylenes”. Journal of the American Chemical Society (на језику: енглески). 90 (8): 2124—2131. ISSN 0002-7863. doi:10.1021/ja01010a034.
- A., Carroll, Felix (2010). Perspectives on structure and mechanism in organic chemistry (2nd изд.). Hoboken, N.J.: John Wiley. ISBN 9780470276105. OCLC 286483846.
- Mootz, Dietrich; Deeg, Axel (јул 1992). „2-Butyne and hydrogen chloride cocrystallized: solid-state geometry of Cl-H.cntdot..cntdot..cntdot..pi. hydrogen bonding to the carbon-carbon triple bond”. Journal of the American Chemical Society (на језику: енглески). 114 (14): 5887—5888. ISSN 0002-7863. doi:10.1021/ja00040a077.
- H., Lowry, Thomas (1987). Mechanism and theory in organic chemistry
 . Richardson, Kathleen Schueller. (3rd изд.). New York: Harper & Row. ISBN 978-0060440848. OCLC 14214254.
. Richardson, Kathleen Schueller. (3rd изд.). New York: Harper & Row. ISBN 978-0060440848. OCLC 14214254. - Wang, Z.; Tu, Y.; Frohn, M.; Zhang, J.; Shi, Y. (1997). „An Efficient Catalytic Asymmetric Epoxidation Method”. J. Am. Chem. Soc. 119 (46): 11224—11235. doi:10.1021/ja972272g.
- Davis, F. A.; Kumar, A.; Chen, B. C. (1991). „Chemistry of oxaziridines. 16. A short, highly enantioselective synthesis of the AB-ring segments of γ-rhodomycionone and α-citromycinone using (+)-[(8,8-dimethoxycamphoryl)sulfonyl]oxaziridine”. J. Org. Chem. 56 (3): 1143—1145. doi:10.1021/jo00003a042.
- Uehlin, L.; Wirth, T. (2001). „Novel Polymer-Bound Chiral Selenium Electrophiles”. Org. Lett. 3 (18): 2931—2933. PMID 11529793. doi:10.1021/ol0164435.
- Tjosaas F, Fiksdahl A (фебруар 2006). „A simple synthetic route to methyl 3-fluoropyridine-4-carboxylate by nucleophilic aromatic substitution”. Molecules (Basel, Switzerland). 11 (2): 130—3. PMC 6148553
 . PMID 17962783. doi:10.3390/11020130
. PMID 17962783. doi:10.3390/11020130  .
. - Bella M, Kobbelgaard S, Jørgensen KA (март 2005). „Organocatalytic regio- and asymmetric C-selective S(N)Ar reactions-stereoselective synthesis of optically active spiro-pyrrolidone-3,3'-oxoindoles”. Journal of the American Chemical Society. 127 (11): 3670—1. PMID 15771481. doi:10.1021/ja050200g.
Спољашње везе
- „Nucleophiles and Electrophiles”. butane.chem.uiuc.edu. Приступљено 2020-09-21.
- „Electrophile | chemistry”. Encyclopedia Britannica (на језику: енглески). Приступљено 2020-09-21.
- Aromatic Substitution Reactions – MSU