Талк — разлика између измена
. ознака: везе до вишезначних одредница |
|||
| Ред 92: | Ред 92: | ||
== Употребе == |
== Употребе == |
||
[[File:Talcum Powder.JPEG|thumb|250п|Talcum powder]] |
|||
[[File:Talc.GIF|thumb|250п|The structure of talc is composed of Si<sub>2</sub>O<sub>5</sub> sheets with magnesium sandwiched between sheets in octahedral sites.]] |
[[File:Talc.GIF|thumb|250п|The structure of talc is composed of Si<sub>2</sub>O<sub>5</sub> sheets with magnesium sandwiched between sheets in octahedral sites.]] |
||
Talc is used in many industries, including paper making, [[plastic]], paint and coatings (e.g. for metal casting molds), rubber, food, electric cable, pharmaceuticals, cosmetics, and ceramics. A coarse grayish-green high-talc rock is [[soapstone]] or [[Soapstone|steatite]], used for stoves, sinks, electrical switchboards, etc. It is often used for surfaces of laboratory table tops and electrical switchboards because of its resistance to heat, electricity and acids. |
Talc is used in many industries, including paper making, [[plastic]], paint and coatings (e.g. for metal casting molds), rubber, food, electric cable, pharmaceuticals, cosmetics, and ceramics. A coarse grayish-green high-talc rock is [[soapstone]] or [[Soapstone|steatite]], used for stoves, sinks, electrical switchboards, etc. It is often used for surfaces of laboratory table tops and electrical switchboards because of its resistance to heat, electricity and acids. |
||
Верзија на датум 19. јун 2022. у 05:50
Један корисник управо ради на овом чланку. Молимо остале кориснике да му допусте да заврши са радом. Ако имате коментаре и питања у вези са чланком, користите страницу за разговор.
Хвала на стрпљењу. Када радови буду завршени, овај шаблон ће бити уклоњен. Напомене
|
| Талк | |
|---|---|
 | |
| Опште информације | |
| Категорија | Силикатни минерал |
| Формула | Mg3Si4O10(OH)2 |
| Струнцова класификација | 9.EC.05 |
| Кристалне системе | Monoclinic or triclinic[1] |
| Кристална класа | Either prismatic (2m) or pinacoidal (1)[2] |
| Просторна група | C2/c or C1 |
| Јединична ћелија | a = 5.291 Å, b = 9.173 Å c = 5.290 Å; α = 98.68° β = 119.90°, γ = 90.09°; Z = 2 or a = 5.287 Å, b = 9.158 Å c = 18.95 [Å], β = 99.3°; Z = 4[2] |
| Идентификација | |
| Боја | Light to dark green, brown, white, grey, colorless |
| Кристални хабитус | Foliated to fibrous masses, rare as platey to pyramidal crystals |
| Цепљивост | Perfect on {001} basal cleavage |
| Прелом | Flat surfaces (not cleavage), fracture in an uneven pattern |
| Чврстина | Sectile |
| Тврдоћа по Мосу | 1 (defining mineral) |
| Сјајност | Waxlike or pearly |
| Огреб | White jot to pearl black |
| Провидност | Translucent |
| Специфична тежина | 2.58 to 2.83 |
| Оптичке особине | Biaxial (-) |
| Индекс преламања | nα = 1.538 – 1.550 nβ = 1.589 – 1.594 nγ = 1.589 – 1.600 |
| Двојно преламање | δ = 0.051 |
| Плеохроизам | Weak in dark varieties |
| Ултравиолетна флуоресценција | Short UV=orange yellow, long UV=yellow |
| Референце | [2][3][4] |
Талк (изведено из персијског преко арапског talq) је минерал по саставу хидратисани магнезијум силикат формуле H2Mg3(SiO3)4 или Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paint, and roofing material. It is a main ingredient in many cosmetics.[5] It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak; though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc is not soluble in water, and is slightly soluble in dilute mineral acids.[6]
Настанак


Талк је матаморфни минерал, дакле настаје метаморфозом магнезијумових минерала, као што су пироксен, амфибол оливин и други слични минерали, у присуству угљендиоксида и воде. Процес је познат као карбонизација талка или стеатизација њим настаје читава серија стена познатих као талкови карбонати.
Талк првенствено настаје хидратацијом и карбонизацијом серпентина, по следећој реакцији:
- + → + +
Талк такође може да настане у реакцији доломита и силицијумдиоксида, which is typical of skarnification of dolomites by silica-flooding in contact metamorphic aureoles:
- + + → + +
Talc can also be formed from magnesian chlorite and quartz in blueschist and eclogite metamorphism by the following metamorphic reaction:
Talc is also found as a diagenetic mineral in sedimentary rocks where it can form from the transformation of metastable hydrated magnesium-clay precursors such as kerolite, sepiolite, or stevensite that can precipitate from marine and lake water in certain conditions.[7]
In this reaction, the ratio of talc and kyanite depends on aluminium content, with more aluminous rocks favoring production of kyanite. This is typically associated with high-pressure, low-temperature minerals such as phengite, garnet, and glaucophane within the lower blueschist facies. Such rocks are typically white, friable, and fibrous, and are known as whiteschist.
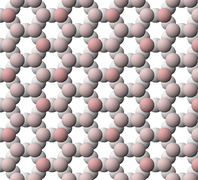
Talc is a trioctahedral layered mineral; its structure is similar to pyrophyllite, but with magnesium in the octahedral sites of the composite layers.[1] The crystal structure of talc is described as TOT, meaning that it is composed of parallel TOT layers weakly bonded to each other by weak van der Waals forces. The TOT layers in turn consist of two tetrahedral sheets (T) strongly bonded to the two faces of a single trioctahedral sheet (O). It is the weak bonding between TOT layers that gives talc its perfect basal cleavage and softness.[8]
The tetrahedral sheets consist of silica tetrahedra, which are silicon ions surrounded by four oxygen ions. The tetrahedra each share three of their four oxygen ions with neighboring tetrahedra to produce a hexagonal sheet. The remaining oxygen ion (the apical oxygen ion) is available to bond with the trioctahedral sheet.[9]
The trioctahedral sheet has the structure of a sheet of the mineral brucite. Apical oxygens take the place of some of the hydroxyl ions that would be present in a brucite sheet, bonding the tetrahedral sheets tightly to the trioctahedral sheet.[10]
Tetrahedral sheets have a negative charge, since their bulk composition is Si4O104-. The trioctahedral sheet has a equal positive charge, since its bulk composition is Mg3(OH)24+ The combined TOT layer thus is electrically neutral.[8]
Because the hexagons in the T and O sheets are slightly different in size, the sheets are slightly distorted when they bond into a TOT layer. This breaks the hexagonal symmetry and reduces it to monoclinic or triclinic symmetry.[11] However, the original hexahedral symmetry is discernible in the pseudotrigonal character of talc crystals.[2]
-
View of tetrahedral sheet structure of talc. The apical oxygen ions are tinted pink.
-
View of trioctahedral sheet of talc. Yellow spheres are hydroxyl; blue are magnesium. Apical oxygen binding sites are white.
-
Talc crystal viewed along the [100] axis, looking along the layers of the crystal
Појава
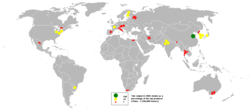
Talc is a common metamorphic mineral in metamorphic belts that contain ultramafic rocks, such as soapstone (a high-talc rock), and within whiteschist and blueschist metamorphic terranes. Prime examples of whiteschists include the Franciscan Metamorphic Belt of the western United States, the western European Alps especially in Italy, certain areas of the Musgrave Block, and some collisional orogens such as the Himalayas, which stretch along Pakistan, India, Nepal, and Bhutan.
Talc carbonate ultramafics are typical of many areas of the Archaean cratons, notably the komatiite belts of the Yilgarn Craton in Western Australia. Talc-carbonate ultramafics are also known from the Lachlan Fold Belt, eastern Australia, from Brazil, the Guiana Shield, and from the ophiolite belts of Turkey, Oman, and the Middle East.
China is the key world talc and steatite producing country with an output of about 2.2M tonnes(2016), which accounts for 30% of total global output. The other major producers are Brazil (12%), India (11%), the U.S. (9%), France (6%), Finland (4%), Italy, Russia, Canada, and Austria (2%, each).[12]
Конфликтни минерал
Extraction in disputed areas of Nangarhar province, Afghanistan, has led the international monitoring group Global Witness to declare talc a conflict resource, as the profits are used to fund armed confrontation between the Taliban and Islamic State.[13]
Употребе

Talc is used in many industries, including paper making, plastic, paint and coatings (e.g. for metal casting molds), rubber, food, electric cable, pharmaceuticals, cosmetics, and ceramics. A coarse grayish-green high-talc rock is soapstone or steatite, used for stoves, sinks, electrical switchboards, etc. It is often used for surfaces of laboratory table tops and electrical switchboards because of its resistance to heat, electricity and acids.
In finely ground form, talc finds use as a cosmetic (talcum powder), as a lubricant, and as a filler in paper manufacture. It is used to coat the insides of inner tubes and rubber gloves during manufacture to keep the surfaces from sticking. Talcum powder, with heavy refinement, has been used in baby powder, an astringent powder used to prevent diaper rash (nappy rash). The American Academy of Pediatrics recommends that parents avoid using baby powder because it poses a risk of respiratory problems, including breathing trouble and serious lung damage if inhaled. The small size of the particles makes it difficult to keep them out of the air while applying the powder. Zinc oxide-based ointments are a much safer alternative.[14]
Soapstone (massive talc) is often used as a marker for welding or metalworking.[15][16]
Talc is also used as food additive or in pharmaceutical products as a glidant. In medicine, talc is used as a pleurodesis agent to prevent recurrent pleural effusion or pneumothorax. In the European Union, the additive number is E553b.
Talc may be used in the processing of white rice as a buffing agent in the polishing stage.
Due to its low shear strength, talc is one of the oldest known solid lubricants. Also a limited use of talc as friction-reducing additive in lubricating oils is made.[17]
| Type | Talc content min. wt% | Loss on ignition at 1000 °C, wt % | Solubility in HCl, max. wt % |
|---|---|---|---|
| A | 95 | 4 – 6.5 | 5 |
| B | 90 | 4–9 | 10 |
| C | 70 | 4–18 | 30 |
| D | 50 | 4–27 | 30 |
Patents are pending on the use of magnesium silicate as a cement substitute. Its production requirements are less energy-intensive than ordinary Portland cement (at a heating requirement of around 650 °C for talc compared to 1500 °C for limestone to produce Portland cement), while it absorbs far more carbon dioxide as it hardens. This results in a negative carbon footprint overall, as the cement substitute removes 0.6 tonnes of CO2 per tonne used. This contrasts with a positive carbon footprint of 0.4 tonne per tonne of conventional cement.[18]
Сигурност
Неколико студија је нашло везу између талка и плућних проблема,[19] рака плућа,[20][21] рака коже и рака јајника.[22]
Види још
Референце
- ^ а б An Introduction to the Rock-Forming Minerals, 2 ed., by W.A. Deer, R.A. Howie, and J. Zussman, 1992, Prentice Hall, ISBN 0-582-30094-0.
- ^ а б в г Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., ур. (1995). „Talc” (PDF). Handbook of Mineralogy. II (Silica, Silicates). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209716.
- ^ Talc. Mindat.org
- ^ Talc. Webmineral
- ^ „Talc”. Minerals Education Coalition.
- ^ Profiles of Drug Substances, Excipients and Related Methodology, Volume 36 ISBN 978-0-123-87667-6 p. 283
- ^ Strauss, Justin V.; MacDonald, Francis A.; Halverson, Galen P.; Tosca, Nicholas J.; Schrag, Daniel P.; Knoll, Andrew H. (2015). „Stratigraphic evolution of the Neoproterozoic Callison Lake Formation: Linking the break-up of Rodinia to the Islay carbon isotope excursion”. American Journal of Science. American Journal of Science (AJS). 315 (10): 881—944. Bibcode:2015AmJS..315..881S. ISSN 0002-9599. S2CID 130671089. doi:10.2475/10.2015.01.
- ^ а б Nesse 2000, стр. 238.
- ^ Nesse 2000, стр. 235.
- ^ Nesse 2000, стр. 235–237.
- ^ Nesse 2000, стр. 239,242.
- ^ Sergeeva, Anna (18. 7. 2018). „China, Brazil, the U.S. and India Remain the Major Consumers on the Global Talc Market”. IndexBox.
- ^ „Talc: the everyday mineral funding Afghan insurgents”. Global Witness. Архивирано из оригинала 24. 5. 2018. г. Приступљено 24. 5. 2018.
- ^ "Is it safe to use baby powder on my baby?". Babycenter.com (2017-05-01). Retrieved on 2017-05-06.
- ^ „Why Do Welders Use Soapstone?”. Welders Manual. Приступљено 7. 3. 2022.
- ^ Young, Pierre. „What Is Soapstone Used for in Welding?”. Welding Headquarters. Приступљено 7. 3. 2022.
- ^ Rudenko, Pavlo; Bandyopadhyay, Amit (2013). „Talc as friction reducing additive to lubricating oil”. Applied Surface Science. 276: 383—389. Bibcode:2013ApSS..276..383R. doi:10.1016/j.apsusc.2013.03.102.
- ^ Jha, Alok (31 December 2008) Revealed: The cement that eats carbon dioxide, The Guardian
- ^ Hollinger (1990). „Pulmonary toxicity of inhaled and intravenous talc.”.
- ^ National Toxicology Program (1993). „NTP Toxicology and Carcinogenesis Studies of Talc (Non-Asbestiform) in Rats and Mice (Inhalation Studies).”.
- ^ NIOSH Worker Notification Program. „Health effects of mining and milling talc.”.(historical)
- ^ Harlow, Cramer Bell (1992). „Perineal exposure to talc and ovarian cancer risk.”. Архивирано из оригинала 03. 03. 2016. г. Приступљено 05. 04. 2020.


![Talc crystal viewed along the [100] axis, looking along the layers of the crystal](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b9/Talc_structure.jpg/180px-Talc_structure.jpg)