Fotohemija

Fotohemija je podoblast hemije koja izučava hemijske reakcije koje se odvijaju uz apsorpciju svetla na atomima ili molekulima.[1] Generalno, ovaj izraz se koristi za opisivanje hemijske reakcije izazvane apsorpcijom ultraljubičastog (talasna dužina od 100 do 400 nm), vidljivog svetla (400–750 nm) ili infracrvenog zračenja (750–2500 nm).[1]
Primeri fotohemijskih reakcija iz svakodnevnog života su fotosinteza, degradacija plastike i formiranje Vitamina D uz pomoć sunčeve svetlosti.[2] Fotohemijske reakcije se odvijaju drugačije od reakcija vođenih temperaturom. Fotohemijski putevi omogućavaju pristup međuproizvodima visoke energije koji se ne mogu generirati toplotno, čime se prevladavaju velike aktivacione barijere u kratkom vremenskom periodu i ostvaruju reakcije koje inače nisu dostupne termičkim procesima. Fotohemija je takođe destruktivna, što ilustruje fotodegradacija plastike.
Koncept[уреди | уреди извор]
Grothas–Drejperov zakon i Štark-Ajnštajnov zakon[уреди | уреди извор]
Fotoekscitacija je prvi korak u fotohemijskom procesu u kome se reaktant podiže u stanje veće energije, u pobuđeno stanje. Prvi zakon fotohemije, poznat kao Grothas–Drejperov zakon (po hemičarima Teodoru Grothasu i Džonu V. Drejperu), navodi da svetlost mora da apsorbuje hemijska supstanca da bi došlo do fotohemijske reakcije. Prema drugom zakonu fotohemije, poznatom kao Štark-Ajnštajnov zakon (po fizičarima Johanesu Štarku i Albertu Ajnštajnu), za svaki foton svetlosti koji apsorbuje hemijski sistem ne aktivira se više od jednog molekula za fotohemijsku reakciju, kako je definisano prema kvantnom prinosu.[3][4]
Fluorescencija i fosforescencija[уреди | уреди извор]
Kada molekul ili atom u osnovnom stanju (S0) apsorbuje svetlost, jedan elektron se pobuđuje na viši orbitalni nivo. Ovaj elektron održava svoj spin prema pravilu odabira spina; drugi prelazi bi kršili zakon očuvanja ugaonog momenta. Ekscitacija do višeg singletnog stanja može biti od HOMO do LUMO ili do više orbite, tako da su singletna pobudna stanja S1, S2, S3… pri različitim energijama moguća.
Kašino pravilo predviđa da bi se viša singletna stanja brzo relaksirala raspadom bez zračenja ili unutrašnjom konverzijom (IC) u S1. Dakle, S1 je obično, ali ne uvek, jedino relevantno singletno pobuđeno stanje. Ovo pobuđeno stanje S1 može dalje da se relaksira do S0 pomoću IC, ali i dozvoljenim radijacionim prelazom iz S1 u S0 koji emituje foton; ovaj proces se naziva fluorescencija.
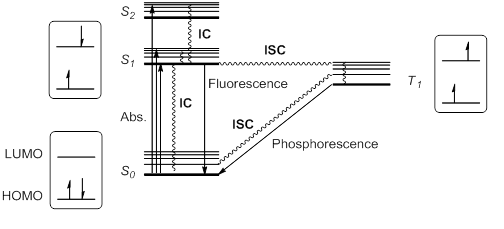
Alternativno, moguće je da pobuđeno stanje S1 doživi inverziju spina i da generiše tripletno uzbuđeno stanje T1 koje ima dva neuparena elektrona sa istim spinom. Ovo kršenje pravila izbora spina moguće je međusistemskim ukrštanjem (ISC) vibracionih i elektronskih nivoa S1 i T1. Prema Hundovom pravilu maksimalne višestrukosti, ovo stanje T1 bilo bi nešto stabilnije od S1.
Ovo tripletno stanje se može opustiti do osnovnog stanja S0 pomoću IC bez zračenja ili putem zračenja koji se naziva fosforescencija. Ovaj proces podrazumeva promenu elektronskog spina, što je zabranjeno pravilima odabira spina, čineći fosforescenciju (od T1 do S0) mnogo sporijom od fluorescencije (od S1 do S0). Dakle, tripletna stanja generalno imaju duži vek trajanja od singletnih. Ovi prelazi se obično sažimaju u dijagram energije stanja ili dijagream Jablonskog, paradigmu molekularne fotohemije.
Ove pobuđene vrste, bilo S1 ili T1, imaju polupraznu niskoenergetsku orbitalu i stoga se znatno više oksiduju od osnovnog stanja. Ali u isto vreme, oni imaju elektron u visokoenergetskoj orbitali, pa se stoga više redukujući. Uopšteno, pobuđene vrste su sklone učestvovanju u procesima prenosa elektrona.[5]
Fotohemija u kombinaciji sa protočnom hemijom[уреди | уреди извор]
Fotohemija sa kontinuiranim tokom nudi višestruke prednosti u odnosu na serijsku fotohemiju. Fotohemijske reakcije su vođene brojem fotona koji su u stanju da aktiviraju molekule izazivajući željenu reakciju. Veliki odnos površine prema zapremini mikroreaktora maksimizira osvetljenje, a istovremeno omogućava efikasno hlađenje, što smanjuje toplotne sporedne proizvode.[6]
Principi[уреди | уреди извор]
Svetlost je tip elektromagnetne radijacije, izvora energije. Po Grotus–Drejperovom zakonu hemijska supstanca mora da absorbuje svetlost da bi došlo do fotohemijske reakcije. Jednim fotonom apsorbovane svetlosti se može aktivirati jedan molekul, ili manje, u zavisnosti od kvantnog prinosa.
U slučaju fotohemijskih reakcija, svetlost daje energiju aktivacije. Jednostavno rečeno, svetlost je jedan mehanizam za obezbeđivanje energije aktivacije potrebne za mnoge reakcije. Ako se koristi laserska svetlost, moguće je selektivno pobuditi molekul tako da se proizvede željeno elektronsko i vibraciono stanje.[7] Jednako tako, emisija iz određenog stanja može se selektivno pratiti, pružajući meru populacije tog stanja. Ako je hemijski sistem pod niskim pritiskom, to omogućava naučnicima da posmatraju raspodelu energije produkata hemijske reakcije pre nego što su razlike u energiji uklonjene i usrednjene usled ponovljenih sudara.
Do hemijske reakcije dolazi kad molekuli poseduju neophodnu energiju aktivacije. Jednostavan primer je sagorevanje benzina (ugljovodonika) do ugljen dioksida i vode. U toj reakciji, energija aktivacije se unosi u obliku toplote ili varnice. Kod fotohemijskih reakcija svetlost pruža energiju aktivacije. Svetlost je jedan od načina davanja energije aktivacije neophodne za mnoge reakcije. Ako se koristi lasersko svetlo, moguće je selektivno pobuditi molekul tako da se proizvede željeno elektronsko i vibraciono stanje. Slično tome, emisija sa specifičnog stanja se može selektivno pratiti, što daje meru zastupljenosti tog stanja. Ako je hemijski sistem na niskom pritisku, moguće je dobiti uvid u distribuciju energije produkata hemijske reakcije pre nego što dođe do disipacije i usrednjavanja energije usled višestrukih molekulskih kolizija.
Apsorpcija svetlosnog fotona na reaktantnom molekulu može da omogući odvijanje reakcije ne samo pružanjem energije aktivacije, nego i promenom simetrije molekulske elektronske konfiguracije, omogućavajući odvijanje inače nepristupačnog reakcionog puta, u skladu sa Vudvord-Hofmanonom pravilima selekcije. 2+2 reakcija cikloadicije je jedan od primera periciklične reakcije koja se može analizirati koristeći ta pravila, ili putem srodnog pristupa molekulsko orbitalne teorije.
Fotohemijske reakcije uzrokuju elektronsku reorganizaciju iniciranu elektromagnetnom radijacijom. Te reakcije su za nekoliko redova veličine brže od termalnih reakcija. Njihova brzina može da bude 10−15 do 10−9 sekundi.
Spektralni regioni[уреди | уреди извор]
Fotohemijske rakcije se tipično izvode koristeći nekoliko specifičnih sekcija elektromagnetskog spektra. Među najčešće korišćenim sekcijama su:
- Ultraljubičasta: 100–400 nm
- Vidljiva: 400–700 nm
- Blisko infracrvena: 700–2500 nm
Fotohemijske reakcije[уреди | уреди извор]
Primeri fotohemijskih reakcija[уреди | уреди извор]
- Fotosinteza: biljke koriste solarnu energiju za pretvaranje ugljen-dioksida i vode u glukozu i kiseonik.
- Ljudsko stvaranje vitamina D izlaganjem sunčevoj svetlosti.
- Bioluminiscencija: npr. kod svitaca enzim u abdomenu katalizuje reakciju koja je proizvodi svetlost.[8]
- Polimerizacije koje su započeli fotoinicijatori, koji se razgrađuju nakon upijanja svetlosti i stvaraju slobodne radikale za polimerizaciju radikalima.
- Fotodegradacija mnogih supstanci, npr. polivinilhlorida i Fp. Bočice sa lekovima se često prave od zatamnjenog stakla kako bi se sprečila fotodegradacija lekova.
- Fotodinamička terapija: svetlost se koristi za uništavanje tumora dejstvom singletnog kiseonika nastalog fotosenzitizovanim reakcijama tripletnog kiseonika. Tipični fotosenzibilizatori uključuju tetrafenilporfirin i metilen plavo. Dobijeni singletni kiseonik je agresivan oksidant, sposoban da pretvori C-H veze u C-OH grupe.
- Diazo proces štampanja
- Tehnologija fotootpora, koja se koristi u proizvodnji mikroelektronskih komponenti.
- Vid se pokreće fotohemijskom reakcijom rodopsina.[9]
- Torajeva fotohemijska proizvodnja e-kaprolaktama.[10]
- Fotohemijska proizvodnja artemisinina, leka protiv malarije.[11][12]
- Fotoalkilacija, koristi se svetlom indukovano dodavanje alkilnih grupa u molekule.
Organska fotohemija[уреди | уреди извор]
Primeri fotohemijskih organskih reakcija su elektrociklične reakcije, reakcije radikala, fotoizomerizacija i Norišove reakcije.[13][14]
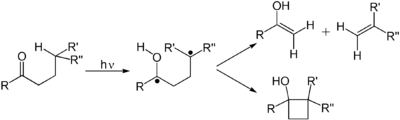
Alkeni podležu mnogim važnim rakcijama koje teku putem fotonski-indukovane tranzicije π do π*. Prvom elektronskom pobuđenom stanju alkena nedostaje π-veza, tako da je rotacija oko C-C veze brza i molekul se uključuje u reakcije koje se ne posmatraju termički. Ove reakcije uključuju cis-trans izomerizaciju, cikloadiciju u drugi (osnovno stanje) alken kako bi se dobili derivati ciklobutana. Cis-trans izomerizacija (poli)alkena se odvija na retinalu, komponenti mašinerije vida. Dimerizacija alkena je relevantna za fotooštećenje DNK, gde se opažaju dimenski timini pri osvetljavanju DNK UV zračenjem. Takvi dimeri ometaju transkripciju. Korisni efekti sunčeve svetlosti povezani su sa fotohemijski indukovanom retrociklizacijom (deciklizacijom) reakcije ergosterola da bi se dobio vitamin D. U Demajovoj reakciji, alken reaguje sa 1,3-diketonom, reagujući preko svog enola, dajući 1,5-diketon. Još jedna uobičajena fotohemijska reakcija je Zimermanovo preuređivanje di-pi-metana.
U industrijskoj primeni, oko 100.000 tona benzil hlorida se godišnje priprema fotohemijskom reakcijom toluena sa hlorom u gasnoj fazi.[15] Molekul hlora apsorbuje svetlost, pri čemu je niska energija ovog prelaza naznačena žućkastom bojom gasa. Foton izaziva homolizu veze Cl-Cl, a nastali radikal hlora pretvara toluen u benzil radikal:
- Cl2 + hν → 2 Cl·
- C6H5CH3 + Cl· → C6H5CH2· + HCl
- C6H5CH2· + Cl· → C6H5CH2Cl
Merkaptani se mogu proizvesti fotohemijskim dodavanjem vodonik sulfida (H2S) u alfa olefine.
Vidi još[уреди | уреди извор]
Reference[уреди | уреди извор]
- ^ а б IUPAC. „photochemistry”. Kompendijum hemijske terminologije (Internet izdanje).
- ^ Glusac, Ksenija (2016). „What has light ever done for chemistry?”. Nature Chemistry. 8 (8): 734—735. Bibcode:2016NatCh...8..734G. PMID 27442273. doi:10.1038/nchem.2582.
- ^ Calvert, J. G.; Pitts, J. N. Photochemistry. Wiley & Sons: New York, US, 1966. Congress Catalog number: 65-24288
- ^ Photochemistry, website of William Reusch (Michigan State University), accessed 26 June 2016
- ^ Wayne, C. E.; Wayne, R. P. Photochemistry, 1st ed.; Oxford University Press: Oxford, United Kingdom, reprinted 2005. ISBN 0-19-855886-4.
- ^ Oelgemöller, Michael; Shvydkiv, Oksana (2011). „Recent Advances in Microflow Photochemistry”. Molecules. 16 (9): 7522—7550. PMC 6264405
 . PMID 21894087. doi:10.3390/molecules16097522.
. PMID 21894087. doi:10.3390/molecules16097522.
- ^ Menzel, Jan P.; Noble, Benjamin B.; Lauer, Andrea; Coote, Michelle L.; Blinco, James P.; Barner-Kowollik, Christopher (2017). „Wavelength Dependence of Light-Induced Cycloadditions”. Journal of the American Chemical Society. 139 (44): 15812—15820. ISSN 0002-7863. PMID 29024596. doi:10.1021/jacs.7b08047. hdl:1885/209117
 .
.
- ^ Saunders, D. S. (2002-11-11). Insect Clocks, Third Edition. стр. 179. ISBN 0444504079.
- ^ Dugave, Christophe (2006-10-06). Cis-trans Isomerization in Biochemistry
 . стр. 56. ISBN 9783527313044.
. стр. 56. ISBN 9783527313044.
- ^ Protti, Stefano; Fagnoni, Maurizio (2009). „The sunny side of chemistry: Green synthesis by solar light”. Photochemical & Photobiological Sciences. 8 (11): 1499—516. PMID 19862408. doi:10.1039/B909128A.
- ^ Peplow, Mark (17. 4. 2013). „Sanofi launches malaria drug production”. Chemistry World.
- ^ Paddon, C. J.; Westfall, P. J.; Pitera, D. J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M. D.; Tai, A.; Main, A.; Eng, D.; Polichuk, D. R. (2013). „High-level semi-synthetic production of the potent antimalarial artemisinin”. Nature (на језику: енглески). 496 (7446): 528—532. ISSN 0028-0836. PMID 23575629. doi:10.1038/nature12051
 .
.
- ^ Klán, Petr; Wirz, Jakob (2009-03-23). Photochemistry of Organic Compounds: From Concepts to Practice. ISBN 978-1405190886.
- ^ Turro, Nicholas J.; Ramamurthy, V.; Scaiano, Juan C. (2010). Modern Molecular Photochemistry of Organic Molecules. ISBN 978-1891389252.
- ^ Rossberg, Manfred; Lendle, Wilhelm; Pfleiderer, Gerhard; Tögel, Adolf; Dreher, Eberhard-Ludwig; Langer, Ernst; Rassaerts, Heinz; Kleinschmidt, Peter; Strack, Heinz; Cook, Richard; Beck, Uwe; Lipper, Karl-August; Torkelson, Theodore R.; Löser, Eckhard; Beutel, Klaus K.; Mann, Trevor (2006). „Chlorinated Hydrocarbons”. Ullmann's Encyclopedia of Industrial Chemistry. ISBN 3527306730. doi:10.1002/14356007.a06_233.pub2.
Literatura[уреди | уреди извор]
- E. J. Bowen, Chemical Aspects of Light. Oxford: The Clarendon Press, 1942. 2nd edition, 1946.
