Rastvaranje

Rastvaranje je proces disperegovanja (ravnomernog razređivanja, raspoređivanja) jedne supstance ili više njih u drugoj. Osim kod pravih smeša, uvek je praćeno razmenom energije sa okolinom.[1][2]
Rastvarač je supstanca koja rastvara rastvorak, čime se formira rastvor. Rastvarač je obično tečnost, ali takođe može biti i čvrsta supstanca, gas ili superkritični fluid. Količina rastvorka koji se može rastvoriti u određenoj zapremini rastvarača varira u zavisnosti od temperature. Uobičajena upotreba organskih rastvarača je u hemijskom čišćenju (npr. tetrahloroetilen), kao sredstvo za razređivanje boje (npr. toluen, terpentin), kao sredstvo za uklanjanje laka za nokte i rastvarač za lepljenje (aceton, metil acetat, etil acetat), u uklanjanju mrlja (npr. heksan, benzin etar), u deterdžentima (citrusni terpeni) i u parfemima (etanol). Voda je rastvarač za polarne molekule i najčešći rastvarač koji koriste živa bića; svi joni i proteini u ćeliji rastvoreni su u vodi unutar ćelije. Rastvarači nalaze razne primene u hemijskoj, farmaceutskoj, naftnoj i gasnoj industriji, uključujući upotrebe u hemijskim sintezama i procesima prečišćavanja.
Rastvarač[uredi | uredi izvor]
Rastvarač, relativan i konvencionalni pojam, naziv za sredinu u kojoj je neka supstanca disperegovana. Idealan rastvor, smeša, bez hemijskog međudejstva sredine i rastvorene supstance ili sa međudejstvom. Može biti čvrst, tečan ili gasovit. Voda je univerzalan rastvarač. Rastvarači kao voda veoma su značajni za život, takođe i u industriji, a naročito važnu ulogu imaju selektivni rastvarači.
Rastvarači u degazaciji[uredi | uredi izvor]
Rastvarači u degazaciji su organska jedinjenja s dvojakom namenom - za rastvaranje degazitora u toku njihove pripreme za upotrebu i za rastvaranje samih bojnih otrova, odnosno za dagazaciju fizičkim putem. Kao rastvarači degazatora najčešće se upotrebljavaju dihloretan, tetrahlorugljenik i titrohloretilen, a kao rastvarači bojnih otrova benzin i petroleum.
Rastvor[uredi | uredi izvor]
Rastvor (čvrst, tečan ili gasovit) nastaje mešanjem dveju ili više supstanci. Zasićen rastvor za datu temperaturu ima maksimalnu koncentraciju rastvorene supstance. Nezasićen rastvor je rastvor u kome se može rastvoriti još izvesna količina već rastvorene supstance sve dok se ne dobije zasićen prostor. Prezasićen prostor je rastvor u kome je rastvoreno više supstanci nego što odgovara zasićenom rastvoru. Nestabilan je i iz njega se lako izdvaja, u obliku taloga, višak rastvorene supstance.
Rastvorljivost[uredi | uredi izvor]
Rastvorljivost neke supstance predstavlja broj grama supstancije koji se rastvara u 100g rastvarača na određenoj temperaturi.
Višekomponentni[uredi | uredi izvor]
Rastvarači[uredi | uredi izvor]
| Ime | Kompozicija |
|---|---|
| Rastvarač 645 | toluen 50%, butil acetat 18%, etil acetat 12%, butanol 10%, etanol 10%. |
| Rastvarač 646 | toluen 50%, etanol 15%, butanol 10%, butil- ili amil acetat 10%, etil celosolv 8%, aceton 7%[3] |
| Rastvarač 647 | butil- ili amil acetat 29,8%, etil acetat 21,2%, butanol 7,7%, toluen ili pirobenzen 41,3%[4] |
| Rastvarač 648 | butil acetat 50%, etanol 10%, butanol 20%, toluen 20%[5] |
| Rastvarač 649 | etil celosolv 30%, butanol 20%, ksilen 50% |
| Rastvarač 650 | etil celosolv 20%, butanol 30%, ksilen 50%[6] |
| Rastvarač 651 | vajt spirit 90%, butanol 10% |
| Rastvarač KR-36 | butil acetat 20%, butanol 80% |
| Rastvarač P-4 | toluen 62%, aceton 26%, butil acetat 12%. |
| Rastvarač P-10 | ksilen 85%, aceton 15%. |
| Rastvarač P-12 | toluen 60%, butil acetat 30%, ksilen 10%. |
| Rastvarač P-14 | cikloheksanon 50%, toluen 50%. |
| Rastvarač P-24 | rastvarač 50%, ksilen 35%, aceton 15%. |
| Rastvarač P-40 | toluen 50%, etil celosolv 30%, aceton 20%. |
| Rastvarač P-219 | toluen 34%, cikloheksanon 33%, aceton 33%. |
| Rastvarač P-3160 | butanol 60%, etanol 40%. |
| Rastvarač RCC | ksilen 90%, butil acetat 10%. |
| Rastvarač RML | etanol 64%, etilcelosolv 16%, toluen 10%, butanol 10%. |
| Rastvarač PML-315 | toluen 25%, ksilen 25%, butil acetat 18%, etil celosolf 17%, butanol 15%. |
| Rastvarač PC-1 | toluen 60%, butil acetat 30%, ksilen 10%. |
| Rastvarač PC-2 | vajt spirit 70%, ksilen 30%. |
| Rastvarač RFG | etanol 75%, butanol 25%. |
| Rastvarač RE-1 | ksilen 50%, aceton 20%, butanol 15%, etanol 15%. |
| Rastvarač RE-2 | rastvarač 70%, etanol 20%, aceton 10%. |
| Rastvarač RE-3 | rastvarač 50%, etanol 20%, aceton 20%, etil celosolv 10%. |
| Rastvarač RE-4 | rastvarač 50%, aceton 30%, etanol 20%. |
| Rastvarač FK-1 (?) | apsolutni alkohol (99,8%) 95%, etil acetat 5% |
Razređivači[uredi | uredi izvor]
| Ime | Kompozicija |
|---|---|
| Razređivač RKB-1 | butanol 50%, ksilen 50% |
| Razređivač RKB-2 | butanol 95%, ksilen 5% |
| Razređivač RKB-3 | ksilen 90%, butanol 10% |
| Razređivač M | etanol 65%, butil acetat 30%, etil acetat 5%. |
| Razređivač P-7 | cikloheksanon 50%, etanol 50%. |
| Razređivač R-197 | ksilen 60%, butil acetat 20%, etil celosolv 20%. |
| Razređivač WFD | toluen 50%, butil acetat (ili amil acetat) 18%, butanol 10%, etanol 10%, etil acetat 9%, aceton 3%. |
Fizička svojstva[uredi | uredi izvor]
Tabela svojstava uobičajenih rastvarača[uredi | uredi izvor]
Rastvarači se grupišu u nepolarne, polarne aprtične, i polarne protične rastvarače, pri čemu svaka grupa ima veću polarnost. Svojstva rastvarača koja nadmašuju svojstva vode su prikazana zadebljanim fontom.
| Rastvarač | Hemijska formula | Tačka ključanja[7] (°C) |
Dielektrična konstanta[8] | Gustina (g/mL) |
Dipolni momenat (D) |
|---|---|---|---|---|---|
Nepolarni rastvarači[uredi | uredi izvor] | |||||
| Pentan | CH3CH2CH2CH2CH3 | 36 | 1,84 | 0,626 | 0,00 |
| Ciklopentan | 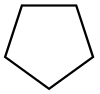 C5H10 |
40 | 1,97 | 0,751 | 0,00 |
| Heksan | CH3CH2CH2CH2CH2CH3 | 69 | 1,88 | 0,655 | 0,00 |
| Cikloheksan |  C6H12 |
81 | 2,02 | 0,779 | 0,00 |
| Benzen | 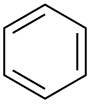 C6H6 |
80 | 2,3 | 0,879 | 0,00 |
| Toluen | C6H5-CH3 | 111 | 2,38 | 0,867 | 0,36 |
| 1,4-Dioksan |  C4H8O2 |
101 | 2,3 | 1,033 | 0,45 |
| Hloroform | CHCl3 | 61 | 4,81 | 1,498 | 1,04 |
| Dietil etar | CH3CH2-O-CH2CH3 | 35 | 4,3 | 0,713 | 1,15 |
| Dihlorometan (DCM) | CH2Cl2 | 40 | 9,1 | 1,3266 | 1,60 |
Polarni aprotični rastvarači[uredi | uredi izvor] | |||||
| Tetrahidrofuran (THF) | 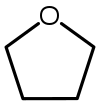 C4H8O |
66 | 7,5 | 0,886 | 1,75 |
| Etil acetat |  CH3-C(=O)-O-CH2-CH3 |
77 | 6,02 | 0,894 | 1,78 |
| Aceton |  CH3-C(=O)-CH3 |
56 | 21 | 0,786 | 2,88 |
| Dimetilformamid (DMF) | 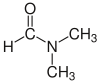 H-C(=O)N(CH3)2 |
153 | 38 | 0,944 | 3,82 |
| Acetonitril (MeCN) | CH3-C≡N | 82 | 37,5 | 0,786 | 3,92 |
| Dimetil sulfoksid (DMSO) |  CH3-S(=O)-CH3 |
189 | 46,7 | 1,092 | 3,96 |
| Nitrometan | CH3-NO2 | 100–103 | 35,87 | 1,1371 | 3,56 |
| Propilen karbonat | C4H6O3 | 240 | 64,0 | 1,205 | 4,9 |
Polarni protični rastvarači[uredi | uredi izvor] | |||||
| Mravlja kiselina |  H-C(=O)OH |
101 | 58 | 1,21 | 1,41 |
| n-Butanol | CH3CH2CH2CH2OH | 118 | 18 | 0,810 | 1,63 |
| Izopropil alkohol (IPA) |  CH3-CH(-OH)-CH3 |
82 | 18 | 0,785 | 1,66 |
| n-Propanol | CH3CH2CH2OH | 97 | 20 | 0,803 | 1,68 |
| Etanol | CH3CH2OH | 79 | 24,55 | 0,789 | 1,69 |
| Metanol | CH3OH | 65 | 33 | 0,791 | 1,70 |
| Sirćetna kiselina |  CH3-C(=O)OH |
118 | 6,2 | 1,049 | 1,74 |
| Voda | H-O-H |
100 | 80 | 1,000 | 1,85 |
Vrednosti parametra Hansenove rastvorljivosti[uredi | uredi izvor]
Vrednosti Hansenovog parametra rastvorljivosti[9][10] zasnivaju se na disperzionim vezama (δD), polarnim vezama (δP) i vodoničnim vezama (δH). One sadrže informacije o međumolekularnim interakcijama sa drugim rastvaračima, kao i sa polimerima, pigmentima, nanočesticama, itd. To omogućava racionalne formulacije znajući, na primer, da postoji dobro podudaranje između rastvarača i polimera. Racionalne supstitucije mogu se praviti tako da se koriste „dobri” rastvarače (efikasni u rastvaranju rastvorka) umesto „loših” (skupih ili opasnih po zdravlje ili životnu sredinu). Sledeća tabela pokazuje da je intuitivno poimanje „nepolarnog”, „polarnog aprotičnog” i „polarnog protičnog” izraženo numerički - „polarni” molekuli imaju viši nivo δP, a protični rastvarači imaju viši nivo δH. Pošto se koriste numeričke vrednosti, poređenje se može racionalno vršiti upoređivanjem brojevima. Na primer, acetonitril je mnogo polarniji od acetona, ali pokazuje nešto slabije vodonično vezivanje.
| Rastvarač | Hemijska formula | δD disperzija | δP polarnost | δH vodonično vezivanje |
|---|---|---|---|---|
Nepolarni rastvarači[uredi | uredi izvor] | ||||
| n-Heksan | CH3CH2CH2CH2CH2CH3 | 14,9 | 0,0 | 0,0 |
| Benzen | C6H6 | 18,4 | 0,0 | 2,0 |
| Toluen | C6H5-CH3 | 18,0 | 1,4 | 2,0 |
| Dietil etar | CH3CH2-O-CH2CH3 | 14,5 | 2,9 | 4,6 |
| Hloroform | CHCl3 | 17,8 | 3,1 | 5,7 |
| 1,4-Dioksan | /-CH2-CH2-O-CH2-CH2-O-\ | 17,5 | 1,8 | 9,0 |
Polarni aprotični rastvarači[uredi | uredi izvor] | ||||
| Etil acetat | CH3-C(=O)-O-CH2-CH3 | 15,8 | 5,3 | 7,2 |
| Tetrahidrofuran (THF) | /-CH2-CH2-O-CH2-CH2-\ | 16,8 | 5,7 | 8,0 |
| Dihlorometan | CH2Cl2 | 17,0 | 7,3 | 7,1 |
| Aceton | CH3-C(=O)-CH3 | 15,5 | 10,4 | 7,0 |
| Acetonitril (MeCN) | CH3-C≡N | 15,3 | 18,0 | 6,1 |
| Dimetilformamid (DMF) | H-C(=O)N(CH3)2 | 17,4 | 13,7 | 11,3 |
| Dimetil sulfoksid (DMSO) | CH3-S(=O)-CH3 | 18,4 | 16,4 | 10,2 |
Polarni protični rastvarači[uredi | uredi izvor] | ||||
| Sirćetna kiselina | CH3-C(=O)OH | 14,5 | 8,0 | 13,5 |
| n-Butanol | CH3CH2CH2CH2OH | 16,0 | 5,7 | 15,8 |
| Izopropanol | CH3-CH(-OH)-CH3 | 15,8 | 6,1 | 16,4 |
| n-Propanol | CH3CH2CH2OH | 16,0 | 6,8 | 17,4 |
| Etanol | CH3CH2OH | 15,8 | 8,8 | 19,4 |
| Metanol | CH3OH | 14,7 | 12,3 | 22,3 |
| Mravlja kiselina | H-C(=O)OH | 14,6 | 10,0 | 14,0 |
| Voda | H-O-H | 15,5 | 16,0 | 42,3 |
Ako je radi zaštite životne sredine ili nekog drugog razloga neophodno da se rastvarač ili mešavina rastvarača zameni drugim ekvivalentnim rastvaračem, zamena se može izvršiti na osnovu njihovih Hansenovih parametara rastvorljivosti. Vrednosti za smeše se uzimaju kao ponderisani proseci vrednosti čistih rastvarača. To se može izračunati pristupom probe i greške, pomoću tabele vrednosti ili HSP softvera.[9][10] Mešavina 1:1 toluena i 1,4 dioksana ima vrednosti δD, δP i δH od 17,8, 1,6 i 5,5, koje su uporedive sa vrednostima hloroforma od 17,8, 3,1 i 5,7 respektivno. Zbog opasnosti po zdravlje povezane sa upotrebom toluena, druge smeše rastvarača se mogu naći pomoću punog HSP seta podataka.
Vidi još[uredi | uredi izvor]
Reference[uredi | uredi izvor]
- ^ Peter Atkins; Julio de Paula (2001). Physical Chemistry (7th izd.). W. H. Freeman. ISBN 0716735393.
- ^ Donald A. McQuarrie; John D. Simon (1997). Physical Chemistry: A Molecular Approach (1st izd.). University Science Books. ISBN 0935702997.
- ^ „dcpt.ru Solvent 646 Characteristics (ru)”. Arhivirano iz originala 26. 10. 2018. g. Pristupljeno 26. 02. 2020.
- ^ „dcpt.ru Solvent 647 Characteristics (ru)”. Arhivirano iz originala 26. 10. 2018. g. Pristupljeno 26. 02. 2020.
- ^ „dcpt.ru Solvent 648 Characteristics (ru)”. Arhivirano iz originala 17. 5. 2017. g. Pristupljeno 26. 2. 2020.
- ^ „dcpt.ru Solvent 650 Characteristics (ru)”. Arhivirano iz originala 26. 10. 2018. g. Pristupljeno 26. 02. 2020.
- ^ Solvent Properties – Boiling Point Arhivirano 2011-06-14 na sajtu Wayback Machine. Xydatasource.com. Retrieved on 26 January 2013.
- ^ Dielectric Constant Arhivirano 2010-07-04 na sajtu Wayback Machine. Macro.lsu.edu. Retrieved on 26 January 2013.
- ^ a b Abbott S, Hansen CM (2008). Hansen solubility parameters in practice. Hansen-Solubility. ISBN 978-0-9551220-2-6.
- ^ a b Hansen CM (januar 2002). Hansen solubility parameters: a user's handbook. CRC press. ISBN 978-0-8493-7248-3.
Literatura[uredi | uredi izvor]
- Lowery TH, Richardson KS (1987). Mechanism and Theory in Organic Chemistry (3rd izd.). Harper Collins Publishers. ISBN 978-0-06-364044-3.
Spoljašnje veze[uredi | uredi izvor]
- "European Solvents Industry Group - ESIG - ESIG European Solvents Industry Group" Solvents in Europe.
- Table and text O-Chem Lecture
- Tables Properties and toxicities of organic solvents
- CDC – Organic Solvents – NIOSH Workplace Safety and Health Topic
- EPA – Solvent Contaminated Wipes
