CBD-DMH
 | |
| IUPAC ime | |
|---|---|
5-(1,1-Dimetilheptil)-2-[(1R,6R)-3-metil-6-(1-metiletenil)-2-cikloheksen-1-il]-1,3-benzenediol | |
| Identifikatori | |
| CAS broj | 97452-63-6 |
| ATC kod | None |
| PubChem | CID 126670 |
| Sinonimi | 1,1-dimetilheptilkanabidiol 5-(1,1-dimetilheptil)-2-(3-metil-6-(1-metiletenil)-2-cikloheksen-1-il)-1,3-benzenediol 5-(1,1-dimetilheptil)kanabidiol 5-(1,1-dimetilheptil)kanabidiol, (1S-trans)-izomer |
| Hemijski podaci | |
| Formula | C25H38O2 |
| Molarna masa | 370.57 |
| |
| |
Kanabidiol-dimetilheptil (CBD-DMH ili DMH-CBD) je sintetički homolog kanabidiola u kome je pentilni lanac zamenjen dimetilheptilnim lancom. Poznato je nekoliko izomera ovog jedinjenja. Izomer koji je najčešće korišćen u istraživanjima je (−)-CBD-DMH. On ima istu stereohemiju kao i prirodni kanabidiol, ali 1,1-dimetilheptilni bočni lanac. Ovo jedinjenje nije psihoaktivno i deluje prvenstveno kao anandamidni inhibitor preuzimanja. Ono je potentnije od kanabidiola kao antikonvulsant i ima približno istu potentnost kao antiinflamatorno sredstvo.[1][2][3][4][5] Neočekivano je utvrđeno da veštački enantiomer (+)-CBD-DMH, koji ima reverznu stereohemiju od kanabidiola, direktno deluje kao agonist kanabinoidnog receptora sa Ki od 17.4nM na CB1 i 211nM na CB2, i proizvodi tipične kanabinoidne efekte u studijama na životinjama.[6]
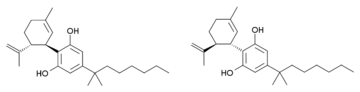
Još jedno blisko srodno jedinjenje je poznato. Ono ima dvostruku vezu u cikloheksenskom prstenu pomerenu na 1,6-poziciju umesto 2,3-pozicije (i.e. analogno je sintetičkim THC analozima, poput paraheksila). Kod tog jedinjenja je izopropenilna grupa zasićena do izopropilne, i ono ima 1,2-dimetilheptilni bočni lanac. Ovo jedinjenje je sintetisano Birčovom redukcijom iz 1,2-dimetilheptilnog analoga kanabidiola. Ono isto tako proizvodi potentne kanabinoidima slične efekte kod životinja, ali ima tri hiralna centra i sastoji se od smeše osam stereoizomera, koji nisu pojedinačno izučavanji, te nije poznato koji su enantiomeri aktivni.[7][8]

Vidi još
[уреди | уреди извор]Reference
[уреди | уреди извор]- ^ Leite, J. R.; Carlini, E. A.; Lander, N.; Mechoulam, R. (1982). „Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs”. Pharmacology. 24 (3): 141—146. PMID 7071126. doi:10.1159/000137588.
- ^ Bisogno, Tiziana; Hanuš, Lumír; De Petrocellis, Luciano; Tchilibon, Susanna; Ponde, Datta E.; Brandi, Ines; Moriello, Aniello Schiano; Davis, John B.; Mechoulam, Raphael; Di Marzo, Vincenzo (2001). „Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide”. British Journal of Pharmacology. 134 (4): 845—852. PMC 1573017
 . PMID 11606325. doi:10.1038/sj.bjp.0704327.
. PMID 11606325. doi:10.1038/sj.bjp.0704327.
- ^ Fride, Ester; Ponde, Datta; Breuer, Aviva; Hanuš, Lumir (2005). „Peripheral, but not central effects of cannabidiol derivatives: Mediation by CB1 and unidentified receptors”. Neuropharmacology. 48 (8): 1117—1129. PMID 15910887. S2CID 16531395. doi:10.1016/j.neuropharm.2005.01.023.
- ^ Ben-Shabat, Shimon; Hanuš, Lumír O.; Katzavian, Galia; Gallily, Ruth (2006). „New Cannabidiol Derivatives: Synthesis, Binding to Cannabinoid Receptor, and Evaluation of Their Antiinflammatory Activity”. Journal of Medicinal Chemistry. 49 (3): 1113—1117. PMID 16451075. doi:10.1021/jm050709m.
- ^ Juknat, Ana; Kozela, Ewa; Kaushansky, Nathali; Mechoulam, Raphael; Vogel, Zvi (2016). „Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol – studies in BV-2 microglia and encephalitogenic T cells”. Journal of Basic and Clinical Physiology and Pharmacology. 27 (3): 289—296. PMID 26540221. S2CID 4829497. doi:10.1515/jbcpp-2015-0071.
- ^ Hanu?, Lum�r O.; Tchilibon, Susanna; Ponde, Datta E.; Breuer, Aviva; Fride, Ester; Mechoulam, Raphael (2005). „Enantiomeric cannabidiol derivatives: Synthesis and binding to cannabinoid receptors”. Organic & Biomolecular Chemistry. 3 (6): 1116. PMID 15750656. doi:10.1039/B416943C. replacement character у
|first1=на позицији 4 (помоћ) - ^ Razdan RK, Pars HG, Thompson WR, Granchelli FE (1974). „Lithium-ammonia reduction of tetrahydrocannabinols”. Tetrahedron Letters. 15 (49–50): 4315—4318. doi:10.1016/S0040-4039(01)92152-5.
- ^ Razdan, K. (1981). „The Total Synthesis of Cannabinoids”. Ур.: Apsimon, John. The Total Synthesis of Natural Products. Wiley Interscience. стр. 245. ISBN 978-0-471-05460-3. OCLC 19487018.
Literatura
[уреди | уреди извор]- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.
