Natrijum hidrogenkarbonat — разлика између измена
м . |
. |
||
| Ред 1: | Ред 1: | ||
{{L|rut}}{{short description|Hemijsko jedinjenje}} |
|||
{{chembox-lat |
|||
{{Chembox-lat |
|||
| Watchedfields = changed |
|||
| Watchedfields = correct |
|||
| verifiedrevid = 311801095 |
|||
| verifiedrevid = 476996774 |
|||
| Name = Natrijum hidrogenkarbonat |
|||
| ImageFile2 = Sodium bicarbonate.jpg |
|||
| ImageName2 = Uzorak natrijum bikarbonata |
|||
| ImageFile = SodiumBicarbonate.svg |
| ImageFile = SodiumBicarbonate.svg |
||
| ImageSize = |
| ImageSize = 121 |
||
| ImageFileL1 = Sodium-3D.png |
| ImageFileL1 = Sodium-3D.png |
||
| ImageNameL1 = Ball and stick model of a sodium cation |
|||
| ImageSizeL1 = 80px |
|||
| ImageFileR1 = Bicarbonate-ion-3D-balls-B.png |
| ImageFileR1 = Bicarbonate-ion-3D-balls-B.png |
||
| ImageNameR1 = Ball and stick model of a bicarbonate anion |
|||
| ImageSizeR1 = 120px |
|||
| IUPACName = Natrijum vodonik karbonat |
|||
| ImageFile2 = Sodium bicarbonate.jpg |
|||
| OtherNames = Prašak za pecivo, bikarb (laboratorijski sleng), bikarbonatna soda, [[nahcolite|nahkolit]] |
|||
| IUPACName = Sodium hydrogen carbonate |
|||
|Section1={{Chembox Identifiers-lat |
|||
| OtherNames = Sodium bicarbonate<br />Bicarbonate of soda<br />Baking soda<br />Sodium hydrogencarbonate<br />[[Nahcolite]] |
|||
| IUPHAR_ligand = 4507 |
|||
| Section1 = {{Chembox Identifiers-lat |
|||
| CASNo = 144-55-8 |
|||
| Abbreviations = |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CASNo = 144-55-8 |
|||
| PubChem = 516892 |
|||
| CASNo_Ref = {{cascite}} |
|||
| |
| ChemSpiderID = 8609 |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 8609 |
|||
| |
| UNII = 8MDF5V39QO |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| EINECS = 205-633-8 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = DB01390 |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = C12603 |
|||
| MeSHName = Sodium+bicarbonate |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 32139 |
|||
| ChEMBL = 1353 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| RTECS = VZ0950000 |
|||
| Beilstein = 4153970 |
|||
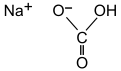
| SMILES = [Na+].OC([O-])=O |
|||
| StdInChI = 1S/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| InChI = 1/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 |
|||
| StdInChIKey = UIIMBOGNXHQVGW-UHFFFAOYSA-M |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| InChIKey = UIIMBOGNXHQVGW-REWHXWOFAQ |
|||
}} |
|||
|Section2={{Chembox Properties-lat |
|||
| Formula = {{chem|Na|HCO|3}} |
|||
| MolarMass = 84,0066 -{g mol}-<sup>−1</sup> |
|||
| Appearance = Beli kristali |
|||
| Odor = bez mirisa |
|||
| Density = {{unbulleted list |
|||
| 2,20 -{g/cm}-<sup>3</sup><ref name=crc>Haynes, p. 4.90</ref> |
|||
}} |
}} |
||
| BoilingPtC = |
|||
| Section2 = {{Chembox Properties-lat |
|||
| |
| MeltingPtC = |
||
| MeltingPt_notes = (razlaže se na [[natrijum karbonat]] počevši od 50 °-{C}-<ref name="crc"/><ref>{{cite journal |doi=10.1007/s10973-006-8182-1 |title=Thermal behaviour of diclofenac, diclofenac sodium and sodium bicarbonate compositions |journal=Journal of Thermal Analysis and Calorimetry |volume=90 |issue=3 |pages=903–907 |year=2007| vauthors = Pasquali I, Bettini R, Giordano F }}</ref><ref>{{cite web |publisher=General Chemistry Online |title=Decomposition of Carbonates |url=http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/carbonate-decomposition.shtml |access-date=28. 06. 2010 |archive-url=https://web.archive.org/web/19991002045519/http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/carbonate-decomposition.shtml |archive-date=02. 10. 1999 |url-status=dead |df= }}</ref>) |
|||
| Na=1|H=1|C=1|O=3 |
|||
| Solubility = {{unbulleted list |
|||
| Appearance = beli kristalni prah |
|||
| 69 -{g/L}- (0 °-{C}-)<ref name=crc3>Haynes, p. 5.194</ref><ref name=UNEP>{{cite web|publisher=[[United Nations Environment Programme]]|url=http://www.chem.unep.ch/irptc/sids/oecdsids/Sodium%20bicarbonate.pdf|title=Sodium Bicarbonate|url-status=dead|archive-url=https://web.archive.org/web/20110516015331/http://www.chem.unep.ch/irptc/sids/oecdsids/Sodium%20bicarbonate.pdf|archive-date=2011-05-16}}</ref> |
|||
| Odor = bez mirisa |
|||
| 96 -{g/L}- (20 °-{C}-)<ref name=crc3/><ref name=UNEP/> |
|||
| Density = 2.173 g/cm<sup>3</sup> |
|||
| |
| 165 -{g/L}- (60 °-{C}-)<ref name=crc3/><ref name=UNEP/> |
||
}} |
|||
| SolubleOther = nerastvoran u [[alkohol]]u, [[etar|etru]] |
|||
| SolubleOther = 0,02 tež.% aceton, 2,13 tež.% metanol @22 °-{C}-.<ref>{{cite journal | title = Solubilities of Sodium Carbonate and Sodium Bicarbonate in Acetone-Water and Methanol-Water Mixtures | journal = J. Chem. Eng. Data | year = 1966 | volume = 11 | issue = 3 | pages = 323–324 | doi = 10.1021/je60030a009| vauthors = Ellingboe JL, Runnels JH }}</ref> nerastvoran u [[etanol]]u |
|||
| MeltingPt = decomp: 323.15 K (50 °C) - 543.15 K (270 °C)<ref>{{cite web |publisher=General Chemistry Online |title=Decomposition of Carbonates |url=http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/carbonate-decomposition.shtml |access-date=28. 06. 2010 |archive-url=https://web.archive.org/web/19991002045519/http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/carbonate-decomposition.shtml |archive-date=02. 10. 1999 |url-status=dead |df= }}</ref> |
|||
| |
| LogP = −0,82 |
||
| pKa = |
| pKa = {{unbulleted list |
||
| 10,329<ref name=crc4>Haynes, p. 7.23</ref> |
|||
| 6,351 (ugljena kiselina)<ref name=crc4/> |
|||
}} |
|||
| RefractIndex = nα = 1,377 nβ = 1,501 nγ = 1,583 |
|||
}} |
|||
|Section3={{Chembox Structure-lat |
|||
| CrystalStruct = Monoclinic |
|||
}} |
|||
|Section6={{Chembox Pharmacology-lat |
|||
| ATCCode_prefix = B05 |
|||
| ATCCode_suffix = CB04 |
|||
| ATC_Supplemental = {{ATC|B05|XA02}}, {{ATCvet|G04|BQ01}} |
|||
| AdminRoutes = Intravenous, oral |
|||
}} |
|||
|Section5={{Chembox Thermochemistry-lat |
|||
| DeltaHf = −950.8 kJ/mol<ref name=crc2>Haynes, p. 5.19</ref> |
|||
| DeltaGf = −851.0 kJ/mol<ref name=crc2/> |
|||
| Entropy = 101.7 J/mol K<ref name=crc2/> |
|||
| HeatCapacity = 87.6 J/mol K<ref name=crc2/> |
|||
}} |
|||
|Section7={{Chembox Hazards-lat |
|||
| ExternalSDS = |
|||
| MainHazards = Uzrokuje ozbilju iritaciju oka |
|||
| NFPA-H = 0 |
|||
| NFPA-F = 0 |
|||
| NFPA-R = 1 |
|||
| LD50 = 4220 -{mg/kg}- (pacov, oralno)<ref>{{cite web |url= https://chem.nlm.nih.gov/chemidplus/rn/144-55-8 | work = ChemIDplus | publisher = U.S. National Library of Medicine | title = Sodium bicarbonate [USP:JAN] | last = Chambers | first = Michael | name-list-format = vanc }}</ref> |
|||
| FlashPt = Incombustible |
|||
}} |
|||
|Section8={{Chembox Related-lat |
|||
| OtherAnions = [[Natrijum karbonat]] |
|||
| OtherCations = {{unbulleted list |
|||
| [[Amonijum bikarbonat]] |
|||
| [[Kalijum bikarbonat]] |
|||
}} |
}} |
||
| OtherCompounds = {{unbulleted list |
|||
| Section7 = {{Chembox Hazards-lat |
|||
| [[Natrijum bisulfat]] |
|||
| ExternalSDS = [http://siri.org/msds/f2/bdm/bdmjw.html External MSDS] |
|||
| [[Natrijum hidrogen fosphat]] |
|||
| MainHazards = |
|||
| NFPA-H = 1 |
|||
| NFPA-F = 0 |
|||
| NFPA-R = 0 |
|||
| FlashPt = ne-zapaljiv |
|||
| RPhrases = |
|||
| SPhrases = |
|||
| LD50 = 4220 mg/kg |
|||
}} |
|||
| Section8 = {{Chembox Related-lat |
|||
| OtherAnions = [[Natrijum karbonat]] |
|||
| OtherCations = [[Kalijum bikarbonat]]<br />[[Amonijum bikarbonat]] |
|||
| OtherCompounds = [[Natrijum bisulfat]]<br />[[Natrijum hidrogen fosphat]] |
|||
}} |
}} |
||
}} |
|||
}} |
}} |
||
[[File:Cupcake2020.jpg|thumb|Pečeni [[kapkejk]]ovi soda bikarbonom kao sredstvom za podizanje]] |
|||
'''Natrijum hidrogenkarbonat''', soda-bikarbona ili natrijum bikarbonat (-{NaHCO}-<sub>3</sub>) je beli prah koji je slabo rastvorljiv u [[voda|vodi]].<ref>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego. {{page|year=2001|isbn=978-0-12-352651-9|pages=}}</ref> |
|||
'''Natrijum hidrogenkarbonat''', soda-bikarbona ili natrijum bikarbonat ([[IUPAC name|IUPAC ime]]: '''natrijum vodonik karbonat''', -{NaHCO}-<sub>3</sub>) je beli prah koji je slabo rastvorljiv u [[voda|vodi]].<ref>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego. {{page|year=2001|isbn=978-0-12-352651-9|pages=}}</ref> It is a [[salt (chemistry)|salt]] composed of a [[sodium]] cation (Na<sup>+</sup>) and a [[bicarbonate]] anion (HCO<sub>3</sub><sup>−</sup>). Sodium bicarbonate is a white solid that is [[crystal]]line, but often appears as a fine powder. It has a slightly salty, [[alkaline]] taste resembling that of washing soda ([[sodium carbonate]]). The natural mineral form is [[nahcolite]]. It is a component of the mineral [[natron]] and is found dissolved in many [[mineral spring]]s. |
|||
== Nomenklatura == |
|||
Because it has long been known and is widely used, the salt has many related names such as '''baking soda''', '''bread soda''', '''cooking soda''', and '''bicarbonate of soda'''. The term ''baking soda'' is more common in the United States, whereas ''bicarbonate of soda'' is more common in Australia and Britain.<ref>{{cite web | title = What's the difference between bicarbonate of soda, baking soda and baking powder? | url = https://www.thatslife.com.au/bicarbonate-of-soda-baking-soda-baking-powder-difference | work = ThatsLife! Pacific Network }}</ref> In colloquial usage, the names sodium bicarbonate and bicarbonate of soda are often truncated; forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common. |
|||
The word ''saleratus'', from [[Latin language|Latin]] ''sal æratus'' (meaning "aerated salt"), was widely used in the [[19th century]] for both sodium bicarbonate and [[potassium bicarbonate]]. |
|||
It is known as one of the [[E number]] food additives '''E500'''. |
|||
The prefix ''bi'' in ''bicarbonate'' comes from an outdated naming system and is based on the observation that there is twice as much [[carbonate]] (CO<sub>3</sub>) per sodium in sodium bicarbonate (NaHCO<sub>3</sub>) as there is in [[sodium carbonate]] (Na<sub>2</sub>CO<sub>3</sub>). The modern chemical formulas of these compounds express their precise chemical compositions (which were unknown when the names ''sodium carbonate'' and ''sodium bicarbonate'' were coined) as sodium hydrogen carbonate (NaHCO<sub>3</sub>) and sodium carbonate (Na<sub>2</sub>CO<sub>3</sub>). These names are unambiguous since sodium always has the +1 [[oxidation state]] and carbonate the −2 oxidation state. |
|||
== Hemijske osobine == |
== Hemijske osobine == |
||
| Ред 70: | Ред 133: | ||
== Upotreba == |
== Upotreba == |
||
Koristi se u proizvodnji [[Prašak za pecivo|praška za pecivo]],<ref name=arm>{{cite web |url=http://www.armhammer.com/basics/magic/#9 |title=Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda |publisher=Armhammer.com |date= |accessdate=30. 7. 2009. |archive-url=https://web.archive.org/web/20090831133032/http://www.armhammer.com/basics/magic/#9 |archive-date=31. 08. 2009 |url-status=dead |df= }}</ref> u kozmetici, i proizvodnji penušavih pića. |
Koristi se u proizvodnji [[Prašak za pecivo|praška za pecivo]],<ref name=arm>{{cite web |url=http://www.armhammer.com/basics/magic/#9 |title=Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda |publisher=Armhammer.com |date= |accessdate=30. 7. 2009. |archive-url=https://web.archive.org/web/20090831133032/http://www.armhammer.com/basics/magic/#9 |archive-date=31. 08. 2009 |url-status=dead |df= }}</ref> u kozmetici, i proizvodnji penušavih pića. |
||
=== Kuvanje === |
|||
==== Narastanje ==== |
|||
In cooking, baking soda is primarily used in [[baking]] as a [[leavening agent]]. When it reacts with acid, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in pancakes, cakes, [[quick bread]]s, [[soda bread]], and other baked and fried foods. The acid-base reaction can be generically represented as follows:<ref>{{cite book | veditors = Bent AJ | title=The Technology of Cake Making | edition=6 | year=1997 | page=102 | publisher=Springer | isbn=9780751403497 | url=https://www.google.com/books?id=OTy8aIWxHhQC&lpg=PP1&pg=PA102#v=onepage&q=&f=false | access-date=2009-08-12}}</ref> |
|||
: -{NaHCO<sub>3</sub> + H<sup>+</sup> → Na<sup>+</sup> + CO<sub>2</sub> + H<sub>2</sub>O}- |
|||
Acidic materials that induce this reaction include [[Monohydrogen phosphate|hydrogen phosphates]], [[cream of tartar]], [[Lemon|lemon juice]], [[yogurt]], [[buttermilk]], [[Cocoa solids|cocoa]], and [[vinegar]]. Baking soda may be used together with [[sourdough]], which is acidic, making a lighter product with a less acidic taste.<ref>{{cite web|url=https://www.uaf.edu/files/ces/publications-db/catalog/hec/FNH-00061.pdf | archive-url = https://web.archive.org/web/20160327162100/https://www.uaf.edu/files/ces/publications-db/catalog/hec/FNH-00061.pdf | archive-date = 27 March 2016|title=Sourdough | first = Julie | last = Cascio | name-list-format = vanc | publisher = University of Alaska Fairbanks Cooperative Extension Service | access-date = 2 May 2017 | id = FNH-00061 }}</ref> |
|||
Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of [[thermal decomposition]], releasing carbon dioxide at temperatures above {{convert|80|C|F|-1}}, as follows:<ref>{{Cite news|url=http://foodreference.about.com/od/Ingredients_Basics/a/What-Is-Baking-Soda.htm|title=The Many Practical Uses of Baking Soda in the Kitchen|newspaper=About.com Food|access-date=2017-01-22|quote=In a nutshell, the [https://www.thankyourbody.com/uses-for-baking-soda/ uses for baking soda] are many: It deodorizes, neutralizes, and cleans all without the toxic mess of most commercial products.}}</ref> |
|||
: -{2 NaHCO<sub>3</sub> → Na<sub>2</sub>CO<sub>3</sub> + H<sub>2</sub>O + CO<sub>2</sub>}- |
|||
When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO<sub>2</sub> is released (one CO<sub>2</sub> molecule is formed for every two equivalents of NaHCO<sub>3</sub>). Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces [[sodium carbonate]], which is strongly alkaline and gives the baked product a bitter, "soapy" taste and a yellow color. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven. |
|||
When adding acid, alkaline ingredients such as whole milk or [[Dutch process chocolate|Dutch-processed]] [[Cocoa solids|cocoa]] are often added to baked foods to avoid an over-acidic taste from the added acid.<ref>{{cite web|url=http://joythebaker.com/2013/10/baking-101-the-difference-between-baking-soda-and-baking-powder|title=Baking 101: The Difference Between Baking Soda and Baking Powder|work=Joy the Baker|access-date=2015-08-04}}</ref> |
|||
==== Prašak za pecivo ==== |
|||
{{main-lat|Prašak za pecivo}} |
|||
[[Prašak za pecivo]], also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients which are activated by the addition of water, without the need for additional acids in the cooking medium.<ref>{{Cite web|url=http://nzic.org.nz/ChemProcesses/food/6D.pdf|title= The Chemistry of Baking | vauthors = Czernohorsky JH, Hooker R |publisher=New Zealand Institute of Chemistry|access-date=2017-01-22|archive-url=https://web.archive.org/web/20161127151812/http://nzic.org.nz/ChemProcesses/food/6D.pdf|archive-date=2016-11-27|url-status=dead}}</ref><ref>{{Cite news|url=http://www.finecooking.com/item/12173/baking-soda-and-baking-powder|title=Baking Soda and Baking Powder|website=FineCooking.com|language=en|access-date=2017-01-22}}</ref><ref>{{cite web |url=http://www.armandhammer.com/FAQ/BakingSoda.aspx |title=Baking Soda FAQs |website=Arm & Hammer Multi-Brand |publisher=Church & Dwight Company |access-date=20 July 2017 |at=What is the difference baking soda and baking powder?|url-status=dead |archive-url=https://web.archive.org/web/20170627082018/http://www.armandhammer.com/FAQ/bakingsoda.aspx |archive-date=27 June 2017 }}</ref> |
|||
Many forms of baking powder contain sodium bicarbonate combined with [[calcium acid phosphate]], [[sodium aluminium phosphate]], or [[cream of tartar]].<ref>{{cite web|url=http://www.cooking.com/recipes-and-more/glossary.aspx?GlossName=Baking+powder|title=Glossary Ingredients|publisher=Cooking.com}}</ref> Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate. |
|||
==== Drugo ==== |
|||
Sodium bicarbonate was sometimes used in cooking green vegetables, as it gives them a bright green colour—which has been described as artificial-looking—due to its reacting with [[chlorophyll]] to produce [[chlorophyllin]].<ref name="Srilakshmi2003">{{cite book| vauthors = Srilakshmi B |title=Food Science|url=https://books.google.com/?id=_pRVkS6nUPEC&pg=PA188|year=2003|publisher=New Age International|isbn=978-81-224-1481-3|page=188}}</ref> However, this tends to affect taste, texture and nutritional content, and is no longer common.<ref>{{cite web |url=https://www.bbc.co.uk/food/bicarbonate_of_soda |title=Bicarbonate of soda recipes |website=BBC Food|date= | last = Sukhadwala | first = Sejal | name-list-format = vanc |access-date= 20 July 2017}}</ref> |
|||
== Vidi još == |
== Vidi još == |
||
| Ред 78: | Ред 167: | ||
== Spoljašnje veze == |
== Spoljašnje veze == |
||
{{ |
{{Commonscat-lat|Sodium bicarbonate}} |
||
{{Commonscat-lat|Sodium bicarbonate}}}} |
|||
* [http://www.b92.net/zdravlje/vesti.php?yyyy=2011&mm=06&nav_id=520071 Sporedne namene sode bikarbone („B92“, 20. jun 2011)] |
* [http://www.b92.net/zdravlje/vesti.php?yyyy=2011&mm=06&nav_id=520071 Sporedne namene sode bikarbone („B92“, 20. jun 2011)] |
||
{{-}} |
|||
{{Jedinjenja natrijuma-lat}} |
{{Jedinjenja natrijuma-lat}} |
||
{{Authority control}} |
|||
{{Portal bar-lat|Hemija}} |
|||
[[Категорија:Једињења натријума]] |
[[Категорија:Једињења натријума]] |
||
Верзија на датум 31. мај 2020. у 23:20
Jedan korisnik upravo radi na ovom članku. Molimo ostale korisnike da mu dopuste da završi sa radom. Ako imate komentare i pitanja u vezi sa člankom, koristite stranicu za razgovor.
Hvala na strpljenju. Kada radovi budu završeni, ovaj šablon će biti uklonjen. Napomene
|
| |||
| |||

| |||
| Nazivi | |||
|---|---|---|---|
| IUPAC naziv
Natrijum vodonik karbonat
| |||
| Drugi nazivi
Prašak za pecivo, bikarb (laboratorijski sleng), bikarbonatna soda, nahkolit
| |||
| Identifikacija | |||
3D model (Jmol)
|
|||
| Bajlštajn | 4153970 | ||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.122 | ||
| EC broj | 205-633-8 | ||
| E-brojevi | E500(ii) (regulator kiselosti, ...) | ||
| KEGG[1] | |||
| MeSH | Sodium+bicarbonate | ||
| RTECS | VZ0950000 | ||
| UNII | |||
| |||
| Svojstva | |||
| NaHCO 3 | |||
| Molarna masa | 84,0066 g mol−1 | ||
| Agregatno stanje | Beli kristali | ||
| Miris | bez mirisa | ||
| Gustina |
| ||
| Tačka topljenja | (razlaže se na natrijum karbonat počevši od 50 °C[4][9][10]) | ||
| Rastvorljivost | 0,02 tež.% aceton, 2,13 tež.% metanol @22 °C.[7] nerastvoran u etanolu | ||
| log P | −0,82 | ||
| Kiselost (pKa) | |||
| Indeks refrakcije (nD) | nα = 1,377 nβ = 1,501 nγ = 1,583 | ||
| Struktura | |||
| Kristalna rešetka/struktura | Monoclinic | ||
| Termohemija | |||
| Specifični toplotni kapacitet, C | 87.6 J/mol K[11] | ||
| Standardna molarna entropija S |
101.7 J/mol K[11] | ||
Std entalpija
formiranja (ΔfH⦵298) |
−950.8 kJ/mol[11] | ||
Gibsova slobodna energija (ΔfG˚)
|
−851.0 kJ/mol[11] | ||
| Farmakologija | |||
| B05CB04 (WHO) B05XA02, QG04 | |||
| Načini upotrebe | Intravenous, oral | ||
| Opasnosti | |||
| Opasnost u toku rada | Uzrokuje ozbilju iritaciju oka | ||
| NFPA 704 | |||
| Tačka paljenja | Incombustible | ||
| Letalna doza ili koncentracija (LD, LC): | |||
LD50 (LD50)
|
4220 mg/kg (pacov, oralno)[12] | ||
| Srodna jedinjenja | |||
Drugi anjoni
|
Natrijum karbonat | ||
Drugi katjoni
|
|||
Srodna jedinjenja
|
|||
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |||
| Reference infokutije | |||

Natrijum hidrogenkarbonat, soda-bikarbona ili natrijum bikarbonat (IUPAC ime: natrijum vodonik karbonat, NaHCO3) je beli prah koji je slabo rastvorljiv u vodi.[13] It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.
Nomenklatura
Because it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda. The term baking soda is more common in the United States, whereas bicarbonate of soda is more common in Australia and Britain.[14] In colloquial usage, the names sodium bicarbonate and bicarbonate of soda are often truncated; forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common.
The word saleratus, from Latin sal æratus (meaning "aerated salt"), was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.
It is known as one of the E number food additives E500.
The prefix bi in bicarbonate comes from an outdated naming system and is based on the observation that there is twice as much carbonate (CO3) per sodium in sodium bicarbonate (NaHCO3) as there is in sodium carbonate (Na2CO3). The modern chemical formulas of these compounds express their precise chemical compositions (which were unknown when the names sodium carbonate and sodium bicarbonate were coined) as sodium hydrogen carbonate (NaHCO3) and sodium carbonate (Na2CO3). These names are unambiguous since sodium always has the +1 oxidation state and carbonate the −2 oxidation state.
Hemijske osobine
Rastvor natrijum bikarbonata reaguje slabo bazno zbog hidrolize.
To je jedan od aditiva kodiran od strane Evropske unije, identifikovan inicijalima E500.
Rastvoren u vodi daje baznu sredinu. Reaguje sa kiselinama i tako razblažen, oslobađa vodu i ugljen-dioksid.
Zbog svoje sposobonsti da reaguje sa kiselinama, koristi se i u farmaceutskoj industriji kao antacid i protiv gorušice.
Prekomernu upotrebu treba izbegavati, jer utiče na pH vrednost želuca. Višak natrijuma povećava krvni pritisak (povećava rizik od hipertenzije i edema).
Dobijanje
Osim Solvejevog postupka, soda bikarbona se dobija i uvođenjem CO2 u vodeni rastvor natrijum karbonata.
Upotreba
Koristi se u proizvodnji praška za pecivo,[15] u kozmetici, i proizvodnji penušavih pića.
Kuvanje
Narastanje
In cooking, baking soda is primarily used in baking as a leavening agent. When it reacts with acid, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in pancakes, cakes, quick breads, soda bread, and other baked and fried foods. The acid-base reaction can be generically represented as follows:[16]
- NaHCO3 + H+ → Na+ + CO2 + H2O
Acidic materials that induce this reaction include hydrogen phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa, and vinegar. Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acidic taste.[17]
Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 °C (180 °F), as follows:[18]
- 2 NaHCO3 → Na2CO3 + H2O + CO2
When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released (one CO2 molecule is formed for every two equivalents of NaHCO3). Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, "soapy" taste and a yellow color. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven.
When adding acid, alkaline ingredients such as whole milk or Dutch-processed cocoa are often added to baked foods to avoid an over-acidic taste from the added acid.[19]
Prašak za pecivo
Prašak za pecivo, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients which are activated by the addition of water, without the need for additional acids in the cooking medium.[20][21][22] Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate, or cream of tartar.[23] Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate.
Drugo
Sodium bicarbonate was sometimes used in cooking green vegetables, as it gives them a bright green colour—which has been described as artificial-looking—due to its reacting with chlorophyll to produce chlorophyllin.[24] However, this tends to affect taste, texture and nutritional content, and is no longer common.[25]
Vidi još
Reference
- ^ Joanne Wixon; Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast. 17 (1): 48—55. doi:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ а б Haynes, p. 4.90
- ^ а б в Haynes, p. 5.194
- ^ а б в „Sodium Bicarbonate” (PDF). United Nations Environment Programme. Архивирано из оригинала (PDF) 2011-05-16. г.
- ^ Ellingboe JL, Runnels JH (1966). „Solubilities of Sodium Carbonate and Sodium Bicarbonate in Acetone-Water and Methanol-Water Mixtures”. J. Chem. Eng. Data. 11 (3): 323—324. doi:10.1021/je60030a009.
- ^ а б Haynes, p. 7.23
- ^ Pasquali I, Bettini R, Giordano F (2007). „Thermal behaviour of diclofenac, diclofenac sodium and sodium bicarbonate compositions”. Journal of Thermal Analysis and Calorimetry. 90 (3): 903—907. doi:10.1007/s10973-006-8182-1.
- ^ „Decomposition of Carbonates”. General Chemistry Online. Архивирано из оригинала 02. 10. 1999. г. Приступљено 28. 06. 2010.
- ^ а б в г Haynes, p. 5.19
- ^ Chambers M. „Sodium bicarbonate [USP:JAN]”. ChemIDplus. U.S. National Library of Medicine.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego. 2001. ISBN 978-0-12-352651-9.
- ^ „What's the difference between bicarbonate of soda, baking soda and baking powder?”. ThatsLife! Pacific Network.
- ^ „Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda”. Armhammer.com. Архивирано из оригинала 31. 08. 2009. г. Приступљено 30. 7. 2009.
- ^ Bent AJ, ур. (1997). The Technology of Cake Making (6 изд.). Springer. стр. 102. ISBN 9780751403497. Приступљено 2009-08-12.
- ^ Cascio J. „Sourdough” (PDF). University of Alaska Fairbanks Cooperative Extension Service. FNH-00061. Архивирано из оригинала (PDF) 27. 3. 2016. г. Приступљено 2. 5. 2017.
- ^ „The Many Practical Uses of Baking Soda in the Kitchen”. About.com Food. Приступљено 2017-01-22. „In a nutshell, the uses for baking soda are many: It deodorizes, neutralizes, and cleans all without the toxic mess of most commercial products.”
- ^ „Baking 101: The Difference Between Baking Soda and Baking Powder”. Joy the Baker. Приступљено 2015-08-04.
- ^ Czernohorsky JH, Hooker R. „The Chemistry of Baking” (PDF). New Zealand Institute of Chemistry. Архивирано из оригинала (PDF) 2016-11-27. г. Приступљено 2017-01-22.
- ^ „Baking Soda and Baking Powder”. FineCooking.com (на језику: енглески). Приступљено 2017-01-22.
- ^ „Baking Soda FAQs”. Arm & Hammer Multi-Brand. Church & Dwight Company. What is the difference baking soda and baking powder?. Архивирано из оригинала 27. 6. 2017. г. Приступљено 20. 7. 2017.
- ^ „Glossary Ingredients”. Cooking.com.
- ^ Srilakshmi B (2003). Food Science. New Age International. стр. 188. ISBN 978-81-224-1481-3.
- ^ Sukhadwala S. „Bicarbonate of soda recipes”. BBC Food. Приступљено 20. 7. 2017.





