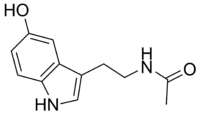
N-Acetilserotonin
| |

| |
| Nazivi | |
|---|---|
| IUPAC naziv
N-[2-(5-hidroksi-1H-indol-3-il)etil]acetamid
| |
| Drugi nazivi
N-acetil-5-hidroksitriptamin, N-acetil-5-HT
| |
| Identifikacija | |
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.560 |
| MeSH | N-Acetylserotonin N-Acetylserotonin |
| |
| Svojstva | |
| C12H14N2O2 | |
| Molarna masa | 218,252 g/mol |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
N-Acetilserotonin (NAS, normelatonin) je prirodni intermedijer u endogenoj produkciji melatonina iz serotonina.[3][4] On se formira iz serotonina posredstvom enzima aralkilaminska N-acetiltransferaza (AANAT) i konvertuje se u melatonin dejstvom acetilserotonin O-metiltransferaze (ASMT). Poput melatonina, NAS je agonist melatoninskih receptora MT1, MT2, i MT3, i može se smatrati neurotransmiterom.[5][6][7][8] Osim toga, NAS je prisutan u pojedinim oblastima mozga gde serotonin and melatonin nisu zastupljeni, iz čega sledi da on ima jedistvene uloge, i da nije samo prekurzor u sintezi melatonina.[5]
Reference[уреди | уреди извор]
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ AXELROD J, WEISSBACH H (1960). „Enzymatic O-methylation of N-acetylserotonin to melatonin”. Science. 131 (3409): 1312. PMID 13795316. doi:10.1126/science.131.3409.1312.
- ^ WEISSBACH H, REDFIELD BG, AXELROD J (1960). „Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin”. Biochimica et Biophysica Acta. 43: 352—3. PMID 13784117. doi:10.1016/0006-3002(60)90453-4.
- ^ а б Jang SW; Liu X; Pradoldej S; et al. (2010). „N-acetylserotonin activates TrkB receptor in a circadian rhythm”. Proceedings of the National Academy of Sciences of the United States of America. 107 (8): 3876. PMC 2840510
 . PMID 20133677. doi:10.1073/pnas.0912531107.
. PMID 20133677. doi:10.1073/pnas.0912531107.
- ^ Zhao H, Poon AM, Pang SF (2000). „Pharmacological characterization, molecular subtyping, and autoradiographic localization of putative melatonin receptors in uterine endometrium of estrous rats”. Life Sciences. 66 (17): 1581—91. PMID 11261588. doi:10.1016/S0024-3205(00)00478-1.
- ^ Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM (1999). „Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists”. British Journal of Pharmacology. 127 (5): 1288—94. PMC 1566130
 . PMID 10455277. doi:10.1038/sj.bjp.0702658.
. PMID 10455277. doi:10.1038/sj.bjp.0702658.
- ^ Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA (1999). „Characterization of 2-[125I]iodomelatonin binding sites in Syrian hamster peripheral organs”. The Journal of Pharmacology and Experimental Therapeutics. 290 (1): 334—40. PMID 10381796. Архивирано из оригинала 15. 12. 2019. г. Приступљено 01. 11. 2012.
