5-Metoksitriptamin
Изглед
 | |
| IUPAC ime | |
|---|---|
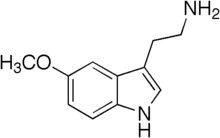
2-(5-Methoxy-1H-indol-3-yl)ethanamine | |
| Klinički podaci | |
| Prodajno ime | Mexamine Meksamin |
| Identifikatori | |
| CAS broj | 608-07-1 |
| PubChem | CID 1833 |
| IUPHAR/BPS | 107 |
| ChemSpider | 1767 |
| KEGG | C05659 |
| ChEMBL | CHEMBL8165 |
| Hemijski podaci | |
| Formula | C11H14N2O |
| Molarna masa | 190.242 g/mol |
| |
| |
5-Metoksitriptamin (5-MT, meksamin) je derivat triptamina. On je blisko srodan neurotransmiterima serotoninu i melatoninu. Za 5-MT je pokazano da se prirodno javlja u telu u niskim nivoima.[1] On je proizvod dekarboksilacije melatonina u epifizi.[1]
5-MT deluje kao pun agonist 5-HT1, 5-HT2, 5-HT4, 5-HT6, i 5-HT7 receptora.[2][3][4][5][6][7][8] On nema afinitet za 5-HT3 receptor. Njegov afinitet za 5-HT1E receptor je veoma slab u poređenju sa drugim 5-HT1 receptorima.[5][9]
Vidi još
[уреди | уреди извор]- 2-Metil-5-hidroksitriptamin
- 5-Benziloksitriptamin
- 5-Karboksamidotriptamin
- α-Metil-5-hidroksitriptamin
Референце
[уреди | уреди извор]- ^ а б Galzin AM, Eon MT, Esnaud H, Lee CR, Pévet P, Langer SZ (1988). „Day-night rhythm of 5-methoxytryptamine biosynthesis in the pineal gland of the golden hamster (Mesocricetus auratus).”. J Endocrinol. 118 (3): 389—397. PMID 2460575. doi:10.1677/joe.0.1180389.
- ^ Wu PH, Gurevich N, Carlen PL (1988). „Serotonin-1A receptor activation in hippocampal CA1 neurons by 8-hydroxy-2-(di-n-propylamino)tetralin, 5-methoxytryptamine and 5-hydroxytryptamine.”. Neurosci Lett. 86 (1): 72—76. PMID 2966313. doi:10.1016/0304-3940(88)90185-1.
- ^ Yamada J, Sugimoto Y, Yoshikawa T, Horisaka K (1997). „Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: involvement of the peripheral 5-HT2A receptor.”. Eur J Pharmacol. 323 (2-3): 235—240. PMID 9128844. doi:10.1016/S0014-2999(97)00029-0.
- ^ Amemiya N, Hatta S, Takemura H, Ohshika H (1996). „Characterization of the contractile response induced by 5-methoxytryptamine in rat stomach fundus strips.”. Eur J Pharmacol. 318 (2-3): 403—409. PMID 9016931. doi:10.1016/S0014-2999(96)00777-7.
- ^ а б Craig DA, Eglen RM, Walsh LK, Perkins LA, Whiting RL, Clarke DE (1990). „5-Methoxytryptamine and 2-methyl-5-hydroxytryptamine-induced desensitization as a discriminative tool for the 5-HT3 and putative 5-HT4 receptors in guinea pig ileum.”. Naunyn Schmiedebergs Arch Pharmacol. 342 (1): 9—16. PMID 2402303.
- ^ Boess FG, Monsma FJ Jr, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ (1997). „Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells.”. Neuropharmacology. 36 (4-5): 713—720. PMID 9225298. doi:10.1016/S0028-3908(97)00019-1.
- ^ Hemedah M, Coupar IM, Mitchelson FJ (1999). „[3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum.”. Br J Pharmacol. 126 (1): 179—188. PMC 1565797
 . PMID 10051134. doi:10.1038/sj.bjp.0702293.
. PMID 10051134. doi:10.1038/sj.bjp.0702293.
- ^ Glennon RA, Dukat M, Westkaemper RB (1. 1. 2000). „Serotonin Receptor Subtypes and Ligands”. American College of Neurophyscopharmacology. Приступљено 11. 4. 2008.
- ^ Roth, Brian (2006). The serotonin receptors. Humana Press. стр. 133. ISBN 978-1-58829-568-2.
Литература
[уреди | уреди извор]- Roth, Brian (2006). The serotonin receptors. Humana Press. стр. 133. ISBN 978-1-58829-568-2.
