Оксидационо стање
Оксидациони број у хемији представља број електрона, које је дати атом предао или примио од других атома док је градио хемијско једињење. Термин „предаја“ или „примање“ електрона у овом случају може да означава потпуну предају електрона другом атому (или групе њих), што доводи до јонске везе, или само делимичну предају електрона, што доводи до поларне ковалентне везе. Ово практично значи да оксидациони број, у ствари, представља број електрона које би атом примио или отпустио када би све везе које је наградио биле јонске.
Оксидациони број се рачуна као баланс свих предатих и примљених електрона у датом атому. Према томе, оксидациони број може бити позитиван (уколико атом отпушта електроне) или негативан (уколико атом прима електроне), као и да има вредност нула (уколико се налази у елементарном стању или су све везе које гради неполарне). Оксидациони бројеви су углавном цели бројеви (мада постоје и једињења у којима поједини елементи за вредности оксидационих бројева немају целобројне вредности).
Дефиниција оксидационог броја од стране IUPAC гласи:[1]
| „ | Оксидациони број: Мера степена оксидације атома у супстанци. Дефинише се као наелектрисање које би атом имао када би се електрони рачунали према утврђеном скупу правила: (1) оксидациони број слободног елемента (несједињеног елемента) је нула; (2) за једноставан (моноатомски) јон, оксидациони број је једнак наелектрисању тог јона; (3) водоник има оксидациони број 1 и кисеоник има оксидациони број -2 у већини једињења. (Изузеци од овога су кад водоник има оксидациони број -1 у хидридима метала, нпр. LiH, а кисеоник има оксидациони број -1 у пероксидима, нпр. H2O2; (4) алгебарски збир оксидационих бројева свих атома у неутралном молекулу мора бити нула, док у јонима алгебарски збир атома мора бити једнак наелектрисању јона. На пример, оксидациони бројеви сумпора у H2S, S8 (елементарни сумпор), SO2, SO3, и H2SO4 су, респективно, -2, 0, +4, +6 и +6. Што је већи оксидациони број датог атома, већи је његов степен оксидације; што је мањи оксидациони број, већи је његов степен редукције. | ” |
Оксидациони број не треба мешати са валенцом. На пример, у једињењу H2O2 кисеоник је двовалентан (сваки атом кисеоника гради по две ковалентне везе), али оксидациони број кисеоника је -1 (јер је једна од тих ковалентних веза између два атома кисеоника, што је чини неполарном).
У називима хемијских једињења, кад елемент може имати више вредности оксидационог броја, оксидациони број се пише римским цифрама у загради, нпр. сумпор(IV)-оксид.
Списак оксидационих стања елемената[уреди | уреди извор]
Ово је листа познатих оксидационих стања хемијских елемената, искључујући неинтегралне вредности. Најчешће присутна стања су подебљана. Табела се заснива на раду Гринвуда и Ирншава[2] са мањим проширењем. Сваки елемент постоји у оксидационом стању 0 када је чист нејонизовани елемент у било којој фази, било да је монатомски или полиатомски алотроп. Колона за оксидационо стање 0 приказује само елементе за које се зна да постоје у оксидационом стању 0 у једињењима.
| Елемент | Негативна стања | Позитивна стања | Група | Напомене | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | |||||
| Z | |||||||||||||||||||
| 1 | водоник | H | −1 | +1 | 1 | ||||||||||||||
| 2 | хелијум | He | 18 | ||||||||||||||||
| 3 | литијум | Li | +1 | 1 | [3] | ||||||||||||||
| 4 | берилијум | Be | 0 | +1 | +2 | 2 | [4][5] | ||||||||||||
| 5 | бор | B | −5 | −1 | 0 | +1 | +2 | +3 | 13 | [6][7][8] | |||||||||
| 6 | угљеник | C | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | |||||||
| 7 | азот | N | −3 | −2 | −1 | +1 | +2 | +3 | +4 | +5 | 15 | ||||||||
| 8 | кисеоник | O | −2 | −1 | 0 | +1 | +2 | 16 | |||||||||||
| 9 | флуор | F | −1 | 17 | |||||||||||||||
| 10 | неон | Ne | 18 | ||||||||||||||||
| 11 | натријум | Na | −1 | +1 | 1 | [3] | |||||||||||||
| 12 | магнезијум | Mg | +1 | +2 | 2 | [9] | |||||||||||||
| 13 | алуминијум | Al | −2 | −1 | +1 | +2 | +3 | 13 | [10][11][12] | ||||||||||
| 14 | силицијум | Si | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [13] | ||||||
| 15 | фосфор | P | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | [14] | ||||||
| 16 | сумпор | S | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | |||||||
| 17 | хлор | Cl | −1 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 17 | [15] | |||||||
| 18 | аргон | Ar | 0 | 18 | [16] | ||||||||||||||
| 19 | калијум | K | −1 | +1 | 1 | [3] | |||||||||||||
| 20 | калцијум | Ca | +1 | +2 | 2 | [17] | |||||||||||||
| 21 | скандијум | Sc | 0 | +1 | +2 | +3 | 3 | [18][19][20] | |||||||||||
| 22 | титанијум | Ti | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 4 | [21][22][23][24] | ||||||||
| 23 | ванадијум | V | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 5 | [22] | |||||||
| 24 | хром | Cr | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [22] | |||||
| 25 | манган | Mn | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | |||||
| 26 | гвожђе | Fe | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 8 | [25][26][27] | ||||
| 27 | кобалт | Co | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 9 | [22] | |||||||
| 28 | никл | Ni | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 10 | [28] | ||||||||
| 29 | бакар | Cu | −2 | 0 | +1 | +2 | +3 | +4 | 11 | [27][29] | |||||||||
| 30 | цинк | Zn | −2 | +1 | +2 | 12 | [27][30] | ||||||||||||
| 31 | галијум | Ga | −5 | −4 | −3 | −2 | −1 | +1 | +2 | +3 | 13 | [11][31][32] | |||||||
| 32 | германијум | Ge | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [33][13] | ||||||
| 33 | арсен | As | −3 | −2 | −1 | +1 | +2 | +3 | +4 | +5 | 15 | [11][34][35] | |||||||
| 34 | селен | Se | −2 | −1 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [36][37][38][39] | |||||||
| 35 | бром | Br | −1 | +1 | +3 | +4 | +5 | +7 | 17 | ||||||||||
| 36 | криптон | Kr | +2 | 18 | |||||||||||||||
| 37 | рубидијум | Rb | −1 | +1 | 1 | [3] | |||||||||||||
| 38 | стронцијум | Sr | +1 | +2 | 2 | [40] | |||||||||||||
| 39 | итријум | Y | 0 | +1 | +2 | +3 | 3 | [41][42][43] | |||||||||||
| 40 | цирконијум | Zr | −2 | +1 | +2 | +3 | +4 | 4 | [22][44] | ||||||||||
| 41 | ниобијум | Nb | −3 | −1 | +1 | +2 | +3 | +4 | +5 | 5 | [22][45] | ||||||||
| 42 | молибден | Mo | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [22] | |||||
| 43 | технецијум | Tc | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 44 | рутенијум | Ru | −4 | −2 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [22][27] | ||||
| 45 | родијум | Rh | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 9 | [22][46] | ||||||
| 46 | паладијум | Pd | 0 | +1 | +2 | +3 | +4 | 10 | [47][48] | ||||||||||
| 47 | сребро | Ag | −2 | −1 | +1 | +2 | +3 | 11 | [27][49] | ||||||||||
| 48 | кадмијум | Cd | −2 | +1 | +2 | 12 | [27][50] | ||||||||||||
| 49 | индијум | In | −5 | −2 | −1 | +1 | +2 | +3 | 13 | [11][51][52] | |||||||||
| 50 | калај | Sn | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [11][53][54][13] | ||||||
| 51 | антимон | Sb | −3 | −2 | −1 | +1 | +2 | +3 | +4 | +5 | 15 | [11][55][56][57] | |||||||
| 52 | телур | Te | −2 | −1 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [11][58][59][60] | |||||||
| 53 | јод | I | −1 | +1 | +3 | +4 | +5 | +6 | +7 | 17 | [61][62] | ||||||||
| 54 | ксенон | Xe | 0 | +2 | +4 | +6 | +8 | 18 | [63][64] | ||||||||||
| 55 | цезијум | Cs | −1 | +1 | 1 | [3] | |||||||||||||
| 56 | баријум | Ba | +1 | +2 | 2 | [65] | |||||||||||||
| 57 | лантан | La | 0 | +1 | +2 | +3 | 3 | [41][66] | |||||||||||
| 58 | церијум | Ce | +2 | +3 | +4 | n/a | |||||||||||||
| 59 | празеодијум | Pr | 0 | +1 | +2 | +3 | +4 | +5 | n/a | [41][67][68][69] | |||||||||
| 60 | неодијум | Nd | 0 | +2 | +3 | +4 | n/a | [41][70] | |||||||||||
| 61 | прометијум | Pm | +2 | +3 | n/a | [71] | |||||||||||||
| 62 | самаријум | Sm | 0 | +2 | +3 | n/a | [41] | ||||||||||||
| 63 | европијум | Eu | +2 | +3 | n/a | ||||||||||||||
| 64 | гадолинијум | Gd | 0 | +1 | +2 | +3 | n/a | [41] | |||||||||||
| 65 | тербијум | Tb | 0 | +1 | +2 | +3 | +4 | n/a | [41][71] | ||||||||||
| 66 | диспрозијум | Dy | 0 | +2 | +3 | +4 | n/a | [41][72] | |||||||||||
| 67 | холмијум | Ho | 0 | +2 | +3 | n/a | [41][71] | ||||||||||||
| 68 | ербијум | Er | 0 | +2 | +3 | n/a | [41][71] | ||||||||||||
| 69 | тулијум | Tm | +2 | +3 | n/a | ||||||||||||||
| 70 | итербијум | Yb | +2 | +3 | n/a | ||||||||||||||
| 71 | лутецијум | Lu | 0 | +2 | +3 | n/a | [41][71] | ||||||||||||
| 72 | хафнијум | Hf | −2 | +1 | +2 | +3 | +4 | 4 | [22][73] | ||||||||||
| 73 | тантал | Ta | −3 | −1 | +1 | +2 | +3 | +4 | +5 | 5 | [22][45] | ||||||||
| 74 | волфрам | W | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [22] | |||||
| 75 | ренијум | Re | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 76 | осмијум | Os | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [27][74] | |||
| 77 | иридијум | Ir | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | 9 | [75][76][77][78] | |||
| 78 | платина | Pt | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 10 | [27][79][80] | |||||
| 79 | злато | Au | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +5 | 11 | [27][81] | |||||||
| 80 | жива | Hg | −2 | +1 | +2 | 12 | [27][82] | ||||||||||||
| 81 | талијум | Tl | −5 | −2 | −1 | +1 | +2 | +3 | 13 | [11][83][84][85] | |||||||||
| 82 | олово | Pb | −4 | −2 | −1 | +1 | +2 | +3 | +4 | 14 | [11][86][87] | ||||||||
| 83 | бизмут | Bi | −3 | −2 | −1 | +1 | +2 | +3 | +4 | +5 | 15 | [88][89][90][91] | |||||||
| 84 | полонијум | Po | −2 | +2 | +4 | +5 | +6 | 16 | [92] | ||||||||||
| 85 | астат | At | −1 | +1 | +3 | +5 | +7 | 17 | |||||||||||
| 86 | радон | Rn | +2 | +6 | 18 | [93][94][95] | |||||||||||||
| 87 | францијум | Fr | +1 | 1 | |||||||||||||||
| 88 | радијум | Ra | +2 | 2 | |||||||||||||||
| 89 | актинијум | Ac | +3 | 3 | |||||||||||||||
| 90 | торијум | Th | +1 | +2 | +3 | +4 | n/a | [96][97] | |||||||||||
| 91 | протактинијум | Pa | +3 | +4 | +5 | n/a | |||||||||||||
| 92 | уранијум | U | +1 | +2 | +3 | +4 | +5 | +6 | n/a | [98][99] | |||||||||
| 93 | нептунијум | Np | +2 | +3 | +4 | +5 | +6 | +7 | n/a | [100] | |||||||||
| 94 | плутонијум | Pu | +2 | +3 | +4 | +5 | +6 | +7 | n/a | [101] | |||||||||
| 95 | америцијум | Am | +2 | +3 | +4 | +5 | +6 | +7 | n/a | [102] | |||||||||
| 96 | киријум | Cm | +3 | +4 | +5 | +6 | n/a | [103][104][105][106] | |||||||||||
| 97 | берклијум | Bk | +3 | +4 | +5 | n/a | [103][104] | ||||||||||||
| 98 | калифорнијум | Cf | +2 | +3 | +4 | +5 | n/a | [103][104] | |||||||||||
| 99 | ајнштајнијум | Es | +2 | +3 | +4 | n/a | [107] | ||||||||||||
| 100 | фермијум | Fm | +2 | +3 | n/a | ||||||||||||||
| 101 | мендељевијум | Md | +2 | +3 | n/a | ||||||||||||||
| 102 | нобелијум | No | +2 | +3 | n/a | ||||||||||||||
| 103 | лоренцијум | Lr | +3 | n/a | |||||||||||||||
| 104 | радерфордијум | Rf | +4 | 4 | |||||||||||||||
| 105 | дубнијум | Db | +5 | 5 | [108] | ||||||||||||||
| 106 | сиборгијум | Sg | 0 | +6 | 6 | [109][110] | |||||||||||||
| 107 | боријум | Bh | +7 | 7 | [111] | ||||||||||||||
| 108 | хасијум | Hs | +8 | 8 | [112] | ||||||||||||||
| 109 | мајтнеријум | Mt | 9 | ||||||||||||||||
| 110 | дармштатијум | Ds | 10 | ||||||||||||||||
| 111 | рендгенијум | Rg | 11 | ||||||||||||||||
| 112 | коперницијум | Cn | +2 | 12 | [113] | ||||||||||||||
| 113 | нихонијум | Nh | 13 | ||||||||||||||||
| 114 | флеровијум | Fl | 14 | ||||||||||||||||
| 115 | московијум | Mc | 15 | ||||||||||||||||
| 116 | ливерморијум | Lv | 16 | ||||||||||||||||
| 117 | тенесин | Ts | 17 | ||||||||||||||||
| 118 | оганесон | Og | 18 | ||||||||||||||||
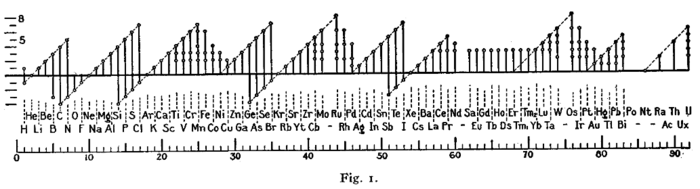
Ране форме (октетно правило)[уреди | уреди извор]
Слику сличног формата је користио Ирвинг Лангмјур 1919. године у једном од раних радова о октетском правилу.[114] Периодичност оксидационих стања била је један од доказа који су навели Лангмјура да усвоји то правило.
Види још[уреди | уреди извор]
Референце[уреди | уреди извор]
- ^ IUPAC Gold Book Архивирано на сајту Wayback Machine (30. март 2016) (језик: енглески)
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (II изд.). Oxford: Butterworth-Heinemann. стр. 27—28. ISBN 0080379419.
- ^ а б в г д Na(−1), K(−1), Rb(−1), and Cs(−1) are known in alkalides; the table by Greenwood and Earnshaw shows −1 only for Na and also erroneously for Li; no lithides are described.
- ^ Be(0) has been observed; see „Beryllium(0) Complex Found”. ChemistryViews. 13. 6. 2016.
- ^ Be(I) has been observed in beryllium monohydride (BeH); see Shayesteh, A.; Tereszchuk, K.; Bernath, P. F.; Colin, R. (2003). „Infrared Emission Spectra of BeH and BeD” (PDF). J. Chem. Phys. 118 (3): 1158. Bibcode:2003JChPh.118.1158S. doi:10.1063/1.1528606. Архивирано из оригинала (PDF) 2. 12. 2007. г. Приступљено 10. 12. 2007.
- ^ B(−5) has been observed in Al3BC, see Schroeder, Melanie. „Eigenschaften von borreichen Boriden und Scandium-Aluminium-Oxid-Carbiden” (PDF) (на језику: немачки). стр. 139. Архивирано из оригинала (PDF) 02. 04. 2015. г. Приступљено 14. 10. 2019.
- ^ B(−1) has been observed in magnesium diboride (MgB2), see Keeler, James; Wothers, Peter (2014). Chemical Structure and Reactivity: An Integrated Approach. Oxford University Press. ISBN 9780199604135.
- ^ B(0) has been observed in diborynes, see Braunschweig, H.; Dewhurst, R. D.; Hammond, K.; Mies, J.; Radacki, K.; Vargas, A. (2012). „Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond”. Science. 336 (6087): 1420—2. Bibcode:2012Sci...336.1420B. PMID 22700924. doi:10.1126/science.1221138.
- ^ Low valent magnesium compounds with Mg(I) have been obtained using bulky ligands; see Green, S. P.; Jones C.; Stasch A. (децембар 2007). „Stable Magnesium(I) Compounds with Mg-Mg Bonds”. Science. 318 (5857): 1754—1757. Bibcode:2007Sci...318.1754G. PMID 17991827. doi:10.1126/science.1150856.
- ^ Al(II) has been observed in aluminium(II) oxide (AlO); see Tyte, D.C. (1964). „Red (B2Π–A2σ) Band System of Aluminium Monoxide”. Nature. 202 (4930): 383—384. Bibcode:1964Natur.202..383T. doi:10.1038/202383a0, and in dialanes (R2Al—AlR2); see Uhl, Werner (2004). „Organoelement Compounds Possessing Al—Al, Ga—Ga, In—In, and Tl—Tl Single Bonds”. Advances in Organometallic Chemistry. Volume 51: 53—108. doi:10.1016/S0065-3055(03)51002-4.
- ^ а б в г д ђ е ж з Negative oxidation states of p-block metals (Al, Ga, In, Sn, Tl, Pb, Bi, Po) and metalloids (Si, Ge, As, Sb, Te, At) may occur in Zintl phases, see: Riedel, Erwin, ур. (2007). Moderne Anorganische Chemie (на језику: немачки). стр. 259, and „Vorlesung Intermetallische Phasen § 6.2 Binäre Zintl-Phasen” (на језику: немачки).
- ^ Al(−2) has been observed in Sr14[Al4]2[Ge]3, see Wemdorff, Marco; Röhr, Caroline (2007). „Sr14[Al4]2[Ge]3: Eine Zintl-Phase mit isolierten [Ge]4–- und [Al4]8–-Anionen / Sr14[Al4]2[Ge]3: A Zintl Phase with Isolated [Ge]4–- and [Al4]8– Anions”. Zeitschrift für Naturforschung B (на језику: немачки). 62 (10): 1227. doi:10.1515/znb-2007-1001.
- ^ а б в „New Type of Zero-Valent Tin Compound”. ChemistryViews. 27. 8. 2016.
- ^ P(0) has been observed, see Wang, Yuzhong; Xie, Yaoming; Wei, Pingrong; King, R. Bruce; Schaefer, Iii; Schleyer, Paul v. R.; Robinson, Gregory H. (2008). „Carbene-Stabilized Diphosphorus”. Journal of the American Chemical Society. 130 (45): 14970—1. PMID 18937460. doi:10.1021/ja807828t.
- ^ The equilibrium Cl2O6⇌2ClO3 is mentioned by Greenwood and Earnshaw, but it has been refuted, see Lopez, Maria; Juan E. Sicre (1990). „Physicochemical properties of chlorine oxides. 1. Composition, ultraviolet spectrum, and kinetics of the thermolysis of gaseous dichlorine hexoxide”. J. Phys. Chem. 94 (9): 3860—3863. doi:10.1021/j100372a094., and Cl2O6 is actually chlorine(V,VII) oxide. However, ClO3 has been observed, see Grothe, Hinrich; Willner, Helge (1994). „Chlorine Trioxide: Spectroscopic Properties, Molecular Structure, and Photochemical Behavior”. Angew. Chem. Int. Ed. 33 (14): 1482—1484. doi:10.1002/anie.199414821.
- ^ Ar(0) has been observed in argon fluorohydride (HArF) and ArCF22+, see Lockyear, J.F.; Douglas, K.; Price, S.D.; Karwowska, M.; et al. (2010). „Generation of the ArCF22+ Dication”. Journal of Physical Chemistry Letters. 1: 358. doi:10.1021/jz900274p.
- ^ Ca(I) has been observed; see Krieck, Sven; Görls, Helmar; Westerhausen, Matthias (2010). „Mechanistic Elucidation of the Formation of the Inverse Ca(I) Sandwich Complex [(thf)3Ca(μ-C6H3-1,3,5-Ph3)Ca(thf)3] and Stability of Aryl-Substituted Phenylcalcium Complexes”. Journal of the American Chemical Society. 132 (35): 12492—501. PMID 20718434. doi:10.1021/ja105534w.
- ^ Sc(0) has been observed; see F. Geoffrey N. Cloke; Karl Khan & Robin N. Perutz (1991). „η-Arene complexes of scandium(0) and scandium(II)”. J. Chem. Soc., Chem. Commun. (19): 1372—1373. doi:10.1039/C39910001372.
- ^ Sc(I) has been observed; see Polly L. Arnold; F. Geoffrey; N. Cloke; Peter B. Hitchcock & John F. Nixon (1996). „The First Example of a Formal Scandium(I) Complex: Synthesis and Molecular Structure of a 22-Electron Scandium Triple Decker Incorporating the Novel 1,3,5-Triphosphabenzene Ring”. J. Am. Chem. Soc. 118 (32): 7630—7631. doi:10.1021/ja961253o.
- ^ Sc(II) has been observed; see Woen, David H.; Chen, Guo P.; Ziller, Joseph W.; Boyle, Timothy J.; Furche, Filipp; Evans, William J. (јануар 2017). „Solution Synthesis, Structure, and CO Reduction Reactivity of a Scandium(II) Complex”. Angewandte Chemie International Edition. 56 (8): 2050—2053. PMID 28097771. doi:10.1002/anie.201611758.
- ^ Ti(I) has been observed in [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2); see Calderazzo, Fausto; Ferri, Isabella; Pampaloni, Guido; Englert, Ulli; Green, Malcolm L. H. (1997). „Synthesis of [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2), the First Titanium(I) Derivatives”. Organometallics. 16 (14): 3100—3101. doi:10.1021/om970155o.
- ^ а б в г д ђ е ж з и ј к Ti(−2), V(−3), Cr(−4), Co(−3), Zr(−2), Nb(−3), Mo(−4), Ru(−2), Rh(−3), Hf(−2), Ta(−3), and W(−4) occur in anionic binary metal carbonyls; see [1], p. 4 (in German); [2], pp. 97–100; [3], p. 239
- ^ Ti(−1) has been reported in [Ti(bipy)3]−, but was later shown to be Ti(+3); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. (2013). „Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study”. Inorganic Chemistry. 52 (4): 2242—56. PMID 23387926. doi:10.1021/ic302799s. However, Ti(−1) occurs in [Ti(η-C6H6]− and [Ti(η-C6H5CH3)]−, see Bandy, J. A.; Berry, A.; Green, M. L. H.; Perutz, R. N.; Prout, K.; Verpeautz, J.-N. (1984). „Synthesis of anionic sandwich compounds: [Ti(η-C6H5R)2]– and the crystal structure of [K(18-crown-6)(µ-H)Mo(η-C5H5)2]”. Inorganic Chemistry. 52 (4): 729—731. doi:10.1039/C39840000729.
- ^ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G., Jr.; Ellis, John E. (2007). „Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)4(S2CNR2)]−”. Chem. Commun. (25): 2639—2641. PMID 17579764. doi:10.1039/B700808B.
- ^ Fe(VII) has been observed in [FeO4]−; see Lu, Jun-Bo; Jian, Jiwen; Huang, Wei; Lin, Hailu; Zhou, Mingfei (2016). „Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−”. Physical Chemistry Chemical Physics. 18 (45): 31125—31131. Bibcode:2016PCCP...1831125L. PMID 27812577. doi:10.1039/C6CP06753K.
- ^ Fe(VIII) has been reported; see Yurii D. Perfiliev; Virender K. Sharma (2008). „Higher Oxidation States of Iron in Solid State: Synthesis and Their Mössbauer Characterization – Ferrates – ACS Symposium Series (ACS Publications)”. Platinum Metals Review. 48 (4): 157—158. doi:10.1595/147106704X10801. However, its existence has been disputed.
- ^ а б в г д ђ е ж з и Fe(−4), Ru(−4), and Os(−4) have been observed in metal-rich compounds containing octahedral complexes [MIn6−xSnx]; Pt(−3) (as a dimeric anion [Pt–Pt]6−), Cu(−2), Zn(−2), Ag(−2), Cd(−2), Au(−2), and Hg(−2) have been observed (as dimeric and monomeric anions; dimeric ions were initially reported to be [T–T]2− for Zn, Cd, Hg, but later shown to be [T–T]4− for all these elements) in La2Pt2In, La2Cu2In, Ca5Au3, Ca5Ag3, Ca5Hg3, Sr5Cd3, Ca5Zn3(structure (AE2+)5(T–T)4−T2−⋅4e−), Yb3Ag2, Ca5Au4, and Ca3Hg2; Au(–3) has been observed in ScAuSn and in other 18-electron half-Heusler compounds. See Changhoon Lee; Myung-Hwan Whangbo (2008). „Late transition metal anions acting as p-metal elements”. Solid State Sciences. 10 (4): 444—449. Bibcode:2008SSSci..10..444K. doi:10.1016/j.solidstatesciences.2007.12.001. and Changhoon Lee; Myung-Hwan Whangbo; Jürgen Köhler (2010). „Analysis of Electronic Structures and Chemical Bonding of Metal-rich Compounds. 2. Presence of Dimer (T–T)4– and Isolated T2– Anions in the Polar Intermetallic Cr5B3-Type Compounds AE5T3 (AE = Ca, Sr; T = Au, Ag, Hg, Cd, Zn)”. Zeitschrift für Anorganische und Allgemeine Chemie. 636 (1): 36—40. doi:10.1002/zaac.200900421.
- ^ Ni(−2) has been observed in Li2[Ni(1,5-COD)2], see Jonas, Klaus (1975). „Dilithium-Nickel-Olefin Complexes. Novel Bimetal Complexes Containing a Transition Metal and a Main Group Metal”. Angew. Chem. Int. Ed. 14 (11): 752—753. doi:10.1002/anie.197507521. and Ellis, John E. (2006). „Adventures with Substances Containing Metals in Negative Oxidation States”. Inorganic Chemistry. 45 (8): 3167—86. PMID 16602773. doi:10.1021/ic052110i.
- ^ Cu(0) has been observed in Cu(tris[2-(diisopropylphosphino)- phenyl]borane), see Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013). „A Polar Copper–Boron One-Electron σ-Bond”. J. Am. Chem. Soc. 135 (10): 3792—3795. PMID 23418750. doi:10.1021/ja4006578.
- ^ Zn(I) has been observed in decamethyldizincocene (Zn2(η5–C5Me5)2); see Resa, I.; Carmona, E.; Gutierrez-Puebla, E.; Monge, A. (2004). „Decamethyldizincocene, a Stable Compound of Zn(I) with a Zn-Zn Bond”. Science. 305 (5687): 1136—8. Bibcode:2004Sci...305.1136R. PMID 15326350. doi:10.1126/science.1101356.
- ^ Ga(−2), Ga(−4), and Ga(−5) have been observed in the magnesium gallides MgGa, Mg2Ga, and Mg5Ga2, respectively; see Patrick Hofmann. „Colture. Ein Programm zur interaktiven Visualisierung von Festkörperstrukturen sowie Synthese, Struktur und Eigenschaften von binären und ternären Alkali- und Erdalkalimetallgalliden” (PDF) (на језику: немачки). стр. 72.
- ^ Ga(−3) has been observed in LaGa, see Dürr, Ines; Bauer, Britta; Röhr, Caroline (2011). „Lanthan-Triel/Tetrel-ide La(Al,Ga)x(Si,Ge)1-x. Experimentelle und theoretische Studien zur Stabilität intermetallischer 1:1-Phasen” (PDF). Z. Naturforsch. (на језику: немачки). 66b: 1107—1121.
- ^ Ge(−1), Ge(−2), and Ge(−3) have been observed in germanides; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). „Germanium”. Lehrbuch der Anorganischen Chemie (на језику: German) (101 изд.). Walter de Gruyter. стр. 953–959. ISBN 978-3-11-012641-9.
- ^ As(I) has been observed in arsenic(I) iodide (AsI); see Ellis, Bobby D.; MacDonald, Charles L. B. (2004). „Stabilized Arsenic(I) Iodide: A Ready Source of Arsenic Iodide Fragments and a Useful Reagent for the Generation of Clusters”. Inorganic Chemistry. 43 (19): 5981—6. PMID 15360247. doi:10.1021/ic049281s.
- ^ As(IV) has been observed in arsenic(IV) hydroxide (As(OH)4) and HAsO-; see Kläning, Ulrik K.; Bielski, Benon H. J.; Sehested, K. (1989). „Arsenic(IV). A pulse-radiolysis study”. Inorganic Chemistry. 28 (14): 2717—24. doi:10.1021/ic00313a007.
- ^ Se(−1) has been observed in diselenides(2−) (Se22−).
- ^ Se(I) has been observed in selenium(I) chloride (Se2Cl2); see „Selenium: Selenium(I) chloride compound data”. WebElements.com. Приступљено 10. 12. 2007.
- ^ Se(III) has been observed in Se2NBr3; see Lau, Carsten; Neumüller, Bernhard; Vyboishchikov, Sergei F.; Frenking, Gernot; Dehnicke, Kurt; Hiller, Wolfgang; Herker, Martin (1996). „Se2NBr3, Se2NCl5, Se2NCl−6: New Nitride Halides of Selenium(III) and Selenium(IV)”. Chemistry: A European Journal. 2 (11): 1393—1396. doi:10.1002/chem.19960021108.
- ^ Se(V) has been observed in SeO2- and HSeO2-; see Kläning, Ulrik K.; Sehested, K. (1986). „Selenium(V). A pulse radiolysis study”. Inorganic Chemistry. 90 (21): 5460—4. doi:10.1021/j100412a112.
- ^ Sr(I) has been observed in strontium monofluoride (SrF); see P. Colarusso; Guo, B.; Zhang, K.-Q.; Bernath, P.F.; et al. (1996). „High-Resolution Infrared Emission Spectrum of Strontium Monofluoride” (PDF). Journal of Molecular Spectroscopy. 175 (1): 158—171. Bibcode:1996JMoSp.175..158C. doi:10.1006/jmsp.1996.0019. Архивирано из оригинала (PDF) 8. 3. 2012. г.
- ^ а б в г д ђ е ж з и ј Yttrium and all lanthanides except Ce, Pm, Eu, Tm, Yb have been observed in the oxidation state 0 in bis(1,3,5-tri-t-butylbenzene) complexes, see Cloke, F. Geoffrey N. (1993). „Zero Oxidation State Compounds of Scandium, Yttrium, and the Lanthanides”. Chem. Soc. Rev. 22: 17—24. doi:10.1039/CS9932200017.
- ^ Y(I) has been observed in yttrium(I) bromide (YBr); see „Yttrium: yttrium(I) bromide compound data”. OpenMOPAC.net. Архивирано из оригинала 23. 7. 2011. г. Приступљено 10. 12. 2007.
- ^ Y(II) has been observed in [(18-crown-6)K][(C5H4SiMe3)3Y]; see MacDonald, M. R.; Ziller, J. W.; Evans, W. J. (2011). „Synthesis of a Crystalline Molecular Complex of Y2+, [(18-crown-6)K][(C5H4SiMe3)3Y]”. J. Am. Chem. Soc. 133 (40): 15914—17. PMID 21919538. doi:10.1021/ja207151y.
- ^ Zr(−1) has been reported in [Zr(bipy)3]− (see Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (II изд.). Oxford: Butterworth-Heinemann. стр. 960. ISBN 0080379419. and Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). „Zirconium”. Lehrbuch der Anorganischen Chemie (на језику: German) (101 изд.). Walter de Gruyter. стр. 1413. ISBN 978-3-11-012641-9.), but was later shown to be Zr(+4); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. (2013). „Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study”. Inorganic Chemistry. 52 (4): 2242—56. PMID 23387926. doi:10.1021/ic302799s.
- ^ а б Nb(I) and Ta(I) occur in CpNb(CO)4 and CpTa(CO)4, see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). „Tantal”. Lehrbuch der Anorganischen Chemie (на језику: German) (101 изд.). Walter de Gruyter. стр. 1430. ISBN 978-3-11-012641-9. and King, R. Bruce (1969). Transition-Metal Organometallic Chemistry: An Introduction. Academic Press. стр. 11. ISBN 978-0-32-315996-8.
- ^ George, G.N.; Klein, S.I.; Nixon, J.F. (1984). „Electron paramagnetic resonance spectroscopic studies on the zero-valent rhodium complex [Rh(P(OPri)3)4] at X-and Q-band frequencies”. Chemical Physics Letters. 108 (6): 627—630. Bibcode:1984CPL...108..627G. doi:10.1016/0009-2614(84)85069-1.
- ^ Pd(I) has been observed; see Crabtree, R. H. (2002). „CHEMISTRY: A New Oxidation State for Pd?”. Science. 295 (5553): 288—289. PMID 11786632. doi:10.1126/science.1067921.
- ^ Pd(III) has been observed; see Powers, D. C.; Ritter, T. (2011). Palladium(III) in Synthesis and Catalysis (PDF). Top. Organomet. Chem. Topics in Organometallic Chemistry. 35. стр. 129—156. Bibcode:2011hoso.book..129P. ISBN 978-3-642-17428-5. PMC 3066514
 . PMID 21461129. doi:10.1007/978-3-642-17429-2_6. Архивирано из оригинала 12. 6. 2013. г.
. PMID 21461129. doi:10.1007/978-3-642-17429-2_6. Архивирано из оригинала 12. 6. 2013. г.
- ^ The Ag− ion has been observed in metal ammonia solutions: see Tran, N. E.; Lagowski, J. J. (2001). „Metal Ammonia Solutions: Solutions Containing Argentide Ions”. Inorganic Chemistry. 40 (5): 1067—68. doi:10.1021/ic000333x.
- ^ Cd(I) has been observed in cadmium(I) tetrachloroaluminate (Cd2(AlCl4)2); see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). „Cadmium”. Lehrbuch der Anorganischen Chemie (на језику: German) (91–100 изд.). Walter de Gruyter. стр. 1056—1057. ISBN 978-3-11-007511-3.
- ^ In(–5) has been observed in La3InGe, see Guloy, A. M.; Corbett, J. D. (1996). „Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties”. Inorganic Chemistry. 35 (9): 2616—22. doi:10.1021/ic951378e.
- ^ In(−2) has been observed in Na2In, see [4], p. 69.
- ^ Sn(−3) has been observed in [Sn2]6−, e.g. in (Ba2)4+(Mg4)8+Sn4−(Sn2)6−Sn2− (with square (Sn2−)n sheets), see Papoian, Garegin A.; Hoffmann, Roald (2000). „Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks”. Angew. Chem. Int. Ed. 2000 (39): 2408—2448. doi:10.1002/1521-3773(20000717)39:14<2408::aid-anie2408>3.0.co;2-u. Приступљено 23. 2. 2015.
- ^ Sn(I) and Sn(III) have been observed in organotin compounds
- ^ Sb(−2) has been observed in [Sb2]4−, e.g. in RbBa4[Sb2][Sb][O], see Boss, Michael; Petri, Denis; Pickhard, Frank; Zönnchen, Peter; Röhr, Caroline (2005). „Neue Barium-Antimonid-Oxide mit den Zintl-Ionen [Sb]3−, [Sb2]4− und 1∞[Sbn]n− / New Barium Antimonide Oxides containing Zintl Ions [Sb]3−, [Sb2]4− and 1∞[Sbn]n−”. Zeitschrift für Anorganische und Allgemeine Chemie (на језику: немачки). 631 (6–7): 1181—1190. doi:10.1002/zaac.200400546.
- ^ Sb(I) and Sb(II) have been observed in organoantimony compounds; for Sb(I), see Šimon, Petr; de Proft, Frank; Jambor, Roman; Růžička, Aleš; Dostál, Libor (2010). „Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures”. Angewandte Chemie International Edition. 49 (32): 5468—5471. PMID 20602393. doi:10.1002/anie.201002209.
- ^ Sb(IV) has been observed in [SbCl]2−
, see Nobuyoshi Shinohara; Masaaki Ohsima (2000). „Production of Sb(IV) Chloro Complex by Flash Photolysis of the Corresponding Sb(III) and Sb(V) Complexes in CH3CN and CHCl3”. Bulletin of the Chemical Society of Japan. 73 (7): 1599—1604. doi:10.1246/bcsj.73.1599. - ^ Te(I) has been observed in tellurium iodide (TeI), see „Tellurium: tellurium iodide”. WebElements.com. Приступљено 23. 2. 2015.
- ^ Te(III) has been observed in [Te(N(SiMe3)2)2]+, see Heinze, Thorsten; Roesky, Herbert W.; Pauer, Frank; Stalke, Dietmar; Sheldrick, George M. (1991). „Synthesis and Structure of the First Tellurium(III) Radical Cation”. Angewandte Chemie International Edition. 30 (12): 1678. doi:10.1002/anie.199116771. Приступљено 23. 2. 2015.
- ^ Te(V) is mentioned by Greenwood and Earnshaw, but they do not give any example of a Te(V) compound. What was long thought to be ditellurium decafluoride (Te2F10) is actually bis(pentafluorotelluryl) oxide, F5TeOTeF5: see Watkins, P. M. (1974). „Ditellurium decafluoride - A Continuing Myth”. Journal of Chemical Education. 51 (9): 520—521. Bibcode:1974JChEd..51..520W. doi:10.1021/ed051p520. However, Te(V) has been observed in HTeO-, TeO-, HTeO2-, and TeO3-; see Kläning, Ulrik K.; Sehested, K. (2001). „Tellurium(V). A Pulse Radiolysis Study”. The Journal of Physical Chemistry A. 105 (27): 6637—45. Bibcode:2001JPCA..105.6637K. doi:10.1021/jp010577i.
- ^ I(IV) has been observed in iodine dioxide (IO2); see Pauling, Linus (1988). „Oxygen Compounds of Nonmetallic Elements”. General Chemistry (3rd изд.). Dover Publications, Inc. стр. 259. ISBN 978-0-486-65622-9.
- ^ I(VI) has been observed in IO3, IO42−, H5IO6−, H2IO52−, H4IO62−, and HIO53−; see Kläning, Ulrik K.; Sehested, Knud; Wolff, Thomas (1981). „Laser flash photolysis and pulse radiolysis of iodate and periodate in aqueous solution. Properties of iodine(VI)”. J. Chem. Soc., Faraday Trans. 1. 77 (7): 1707—18. doi:10.1039/F19817701707.
- ^ Xe(0) has been observed in tetraxenonogold(II) (AuXe42+).
- ^ Xe(I) has been reported in xenon hexafluoroplatinate and xenon hexafluororhodate (see Pauling, Linus (1988). General Chemistry (3rd изд.). Dover Publications, Inc. стр. 250. ISBN 978-0-486-65622-9.), however these compounds were later found to contain Xe(II).
- ^ Ba(I) has been observed in barium monofluoride (BaF); see P. Colarusso; Guo, B.; Zhang, K.-Q.; Bernath, P.F.; et al. (1995). „High-Resolution Fourier Transform Infrared Emission Spectrum of Barium Monofluoride” (PDF). Journal of Molecular Spectroscopy. 170: 59. Bibcode:1996JMoSp.175..158C. doi:10.1006/jmsp.1996.0019. Архивирано из оригинала (PDF) 10. 3. 2005. г.
- ^ La(I) has been observed in lanthanum monohydride (LaH); see Ram, R. S.; Bernath, P. F. (1996). „Fourier Transform Emission Spectroscopy of New Infrared Systems of LaH and LaD” (PDF). Journal of Molecular Spectroscopy. 104 (17): 6444. Bibcode:1996JChPh.104.6444R. doi:10.1063/1.471365. Архивирано из оригинала (PDF) 10. 3. 2005. г.
- ^ Pr(I) has been observed in [PrB4]−; see Chen, Xin; Chen, Teng-Teng; Li, Wang-Lu; Lu, Jun-Bo; Zhao, Li-Juan; Jian, Tian; Hu, Han-Shi; Wang, Lai-Sheng; Li, Jun (13. 12. 2018). „Lanthanides with Unusually Low Oxidation States in the PrB3– and PrB4– Boride Clusters”. Inorganic Chemistry. 58 (1): 411—418. PMID 30543295. doi:10.1021/acs.inorgchem.8b02572.
- ^ Pr(V) has been observed in [PrO2]+; see Zhang, Qingnan; Hu, Shu-Xian; Qu, Hui; Su, Jing; Wang, Guanjun; Lu, Jun-Bo; Chen, Mohua; Zhou, Mingfei; Li, Jun (6. 6. 2016). „Pentavalent Lanthanide Compounds: Formation and Characterization of Praseodymium(V) Oxides”. Angewandte Chemie International Edition. 55 (24): 6896—6900. ISSN 1521-3773. PMID 27100273. doi:10.1002/anie.201602196.
- ^ Hu, Shu-Xian; Jian, Jiwen; Su, Jing; Wu, Xuan; Li, Jun; Zhou, Mingfei (2017). „Pentavalent lanthanide nitride-oxides: NPrO and NPrO− complexes with N≡Pr triple bonds”. Chemical Science (на језику: енглески). 8 (5): 4035—4043. ISSN 2041-6520. PMC 5434915
 . PMID 28580119. doi:10.1039/C7SC00710H.
. PMID 28580119. doi:10.1039/C7SC00710H.
- ^ Nd(IV) has been observed in unstable solid state compounds; see Holleman A. F.; Wiberg E. (2001). Inorganic Chemistry (1st изд.). San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ а б в г д All the lanthanides (La–Lu) in the +2 oxidation state have been observed (except La, Gd, Lu) in dilute, solid solutions of dihalides of these elements in alkaline earth dihalides (see Holleman A. F.; Wiberg E. (2001). Inorganic Chemistry (1st изд.). San Diego: Academic Press. ISBN 0-12-352651-5.) and (except Pm) in organometallic molecular complexes, see Lanthanides Topple Assumptions and Meyer, G. (2014). „All the Lanthanides Do It and Even Uranium Does Oxidation State +2”. Angewandte Chemie International Edition. 53 (14): 3550—51. PMID 24616202. doi:10.1002/anie.201311325.. Additionally, all the lanthanides (La–Lu) form dihydrides (LnH2), dicarbides (LnC2), monosulfides (LnS), monoselenides (LnSe), and monotellurides (LnTe), but for most elements these compounds have Ln3+ ions with electrons delocalized into conduction bands, e. g. Ln3+(H−)2(e−).
- ^ Dy(IV) has been observed in unstable solid state compounds; see Holleman A. F.; Wiberg E. (2001). Inorganic Chemistry (1st изд.). San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Hf(I) has been observed in hafnium monobromide (HfBr), see Marek, G.S.; Troyanov, S.I.; Tsirel'nikov, V.I. (1979). „Кристаллическое строение и термодинамические характеристики монобромидов циркония и гафния / Crystal structure and thermodynamic characteristics of monobromides of zirconium and hafnium”. Журнал неорганической химии / Russian Journal of Inorganic Chemistry (на језику: руски). 24 (4): 890—893.
- ^ Os(−1) has been observed in Na[Os(CO)
13]; see Krause, J.; Siriwardane, Upali; Salupo, Terese A.; Wermer, Joseph R.; Knoeppel, David W.; Shore, Sheldon G. (1993). „Preparation of [Os3(CO)11]2− and its reactions with Os3(CO)12; structures of [Et4N] [HOs3(CO)11] and H2OsS4(CO)”. Journal of Organometallic Chemistry. 454: 263—271. doi:10.1016/0022-328X(93)83250-Y. and Carter, Willie J.; Kelland, John W.; Okrasinski, Stanley J.; Warner, Keith E.; Norton, Jack R. (1982). „Mononuclear hydrido alkyl carbonyl complexes of osmium and their polynuclear derivatives”. Inorganic Chemistry. 21 (11): 3955—3960. doi:10.1021/ic00141a019. - ^ Ir(−3) has been observed in Ir(CO)33−; see Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (II изд.). Oxford: Butterworth-Heinemann. стр. 1117. ISBN 0080379419.
- ^ Ir(VII) has been observed in [(η2-O2)IrO2]+; see C&EN: Iridium dressed to the nines.
- ^ Ir(VIII) has been observed in iridium tetroxide (IrO4); see Gong, Yu; Zhou, Mingfei; Kaupp, Martin; Riedel, Sebastian (2009). „Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII”. Angewandte Chemie International Edition. 48 (42): 7879—7883. PMID 19593837. doi:10.1002/anie.200902733.
- ^ Ir(IX) has been observed in IrO+; see Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary G.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (21. 8. 2014). „Identification of an iridium-containing compound with a formal oxidation state of IX”. Nature. 514 (7523): 475—477. Bibcode:2014Natur.514..475W. PMID 25341786. doi:10.1038/nature13795.
- ^ Pt(−1) and Pt(−2) have been observed in the barium platinides Ba2Pt and BaPt, respectively: see Karpov, Andrey; Konuma, Mitsuharu; Jansen, Martin (2006). „An experimental proof for negative oxidation states of platinum: ESCA-measurements on barium platinides”. Chemical Communications (8): 838—840. PMID 16479284. doi:10.1039/b514631c.
- ^ Pt(I) and Pt(III) have been observed in bimetallic and polymetallic species; see Kauffman, George B.; Thurner, Joseph J.; Zatko, David A. (1967). Ammonium Hexachloroplatinate(IV). Inorganic Syntheses. 9. стр. 182—185. ISBN 978-0-470-13240-1. doi:10.1002/9780470132401.ch51.
- ^ Au(0) has been observed, see Mézaille, Nicolas; Avarvari, Narcis; Maigrot, Nicole; Ricard, Louis; Mathey, François; Le Floch, Pascal; Cataldo, Laurent; Berclaz, Théo; Geoffroy, Michel (1999). „Gold(I) and Gold(0) Complexes of Phosphinine‐Based Macrocycles”. Angewandte Chemie International Edition (21): 3194—3197. doi:10.1002/(SICI)1521-3773(19991102)38:21<3194::AID-ANIE3194>3.0.CO;2-O.
- ^ Hg(IV) has been reported in mercury(IV) fluoride (HgF4); see Xuefang Wang; Lester Andrews; Sebastian Riedel; Martin Kaupp (2007). „Mercury Is a Transition Metal: The First Experimental Evidence for HgF4”. Angew. Chem. Int. Ed. 46 (44): 8371—8375. PMID 17899620. doi:10.1002/anie.200703710. However, it could not be confirmed by later experiments; see Is mercury a transition metal? Архивирано 2016-10-12 на сајту Wayback Machine
- ^ Tl(−5) has been observed in Na23K9Tl15.3, see Dong, Z.-C.; Corbett, J. D. (1996). „Na23K9Tl15.3: An Unusual Zintl Compound Containing Apparent Tl57−, Tl48−, Tl37−, and Tl5− Anions”. Inorganic Chemistry. 35 (11): 3107—12. doi:10.1021/ic960014z.
- ^ Tl(−1) has been observed in caesium thallide (CsTl); see King, R. B.; Schleyer, R. (2004). „Theory and concepts in main-group cluster chemistry”. Ур.: Driess, M.; Nöth, H. Molecular clusters of the main group elements. Wiley-VCH, Chichester. стр. 19. ISBN 978-3-527-61437-0.
- ^ Tl(+2) has been observed in tetrakis(hypersilyl)dithallium ([(Me3Si)Si]2Tl—Tl[Si(SiMe3)]2), see Sonja Henkel; Dr. Karl Wilhelm Klinkhammer; Dr. Wolfgang Schwarz (1994). „Tetrakis(hypersilyl)dithallium(Tl—Tl): A Divalent Thallium Compound”. Angew. Chem. Int. Ed. 33 (6): 681—683. doi:10.1002/anie.199406811.
- ^ Pb(−2) has been observed in BaPb, see Ferro, Riccardo (2008). Nicholas C. Norman, ур. Intermetallic Chemistry. Elsevier. стр. 505. ISBN 978-0-08-044099-6. and Todorov, Iliya; Sevov, Slavi C. (2004). „Heavy-Metal Aromatic Rings: Cyclopentadienyl Anion Analogues Sn56− and Pb56− in the Zintl Phases Na8BaPb6, Na8BaSn6, and Na8EuSn6”. Inorganic Chemistry. 43 (20): 6490—94. doi:10.1021/ic000333x.
- ^ Pb(+1) and Pb(+3) have been observed in organolead compounds, e.g. hexamethyldiplumbane Pb2(CH3)6; for Pb(I), see Siew-Peng Chia; Hong-Wei Xi; Yongxin Li; Kok Hwa Lim; Cheuk-Wai So (2013). „A Base-Stabilized Lead(I) Dimer and an Aromatic Plumbylidenide Anion”. Angew. Chem. Int. Ed. 52 (24): 6298—6301. PMID 23629949. doi:10.1002/anie.201301954.
- ^ Bi(−2) and Bi(−1) occur in Zintl phases, e.g. (Ca2+)22[Bi4]4−([Bi2]4−)4[Bi3−]8; see Ponou, Siméon (2006). „Germanides, Germanide-Tungstate Double Salts and Substitution Effects in Zintl Phases”. Technische Universität München. Lehrstuhl für Anorganische Chemie mit Schwerpunkt Neue Materialien. стр. 68.
- ^ Bi(I) has been observed in bismuth monobromide (BiBr) and bismuth monoiodide (BiI); see Godfrey, S. M.; McAuliffe, C. A.; Mackie, A. G.; Pritchard, R. G. (1998). Nicholas C. Norman, ур. Chemistry of arsenic, antimony, and bismuth. Springer. стр. 67—84. ISBN 978-0-7514-0389-3.
- ^ Bi(+2) has been observed in dibismuthines (R2Bi—BiR2), see Arthur J. Ashe III (1990). Thermochromic Distibines and Dibismuthines. Advances in Organometallic Chemistry. 30. стр. 77—97. ISBN 9780120311309. doi:10.1016/S0065-3055(08)60499-2.
- ^ Bi(IV) has been observed; see A. I. Aleksandrov, I. E. Makarov (1987). „Formation of Bi(II) and Bi(IV) in aqueous hydrochloric solutions of Bi(III)”. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science. 36 (2): 217—220. doi:10.1007/BF00959349.
- ^ Po(V) has been observed in dioxidopolonium(1+) (PoO+); see Thayer, John S. (2010). „Relativistic Effects and the Chemistry of the Heavier Main Group Elements”. Relativistic Methods for Chemists. стр. 78. ISBN 978-1-4020-9974-8. doi:10.1007/978-1-4020-9975-5_2.
- ^ Rn(II) has been observed in radon difluoride (RnF2); see Stein, L. (1970). „Ionic Radon Solution”. Science. 168 (3929): 362—4. Bibcode:1970Sci...168..362S. PMID 17809133. doi:10.1126/science.168.3929.362. and Kenneth S. Pitzer (1975). „Fluorides of radon and element 118”. J. Chem. Soc., Chem. Commun. (18): 760b — 761. doi:10.1039/C3975000760b.
- ^ Rn(IV) is reported by Greenwood and Earnshaw, but is not known to exist; see Sykes, A. G. (1998). „Recent Advances in Noble-Gas Chemistry”. Advances in Inorganic Chemistry. 46. Academic Press. стр. 91—93. ISBN 978-0-12-023646-6. Приступљено 22. 11. 2012.
- ^ Rn(VI) is known in radon trioxide (RnO3); see Sykes, A. G. (1998). „Recent Advances in Noble-Gas Chemistry”. Advances in Inorganic Chemistry. 46. Academic Press. стр. 91—93. ISBN 978-0-12-023646-6. Приступљено 22. 11. 2012.
- ^ Th(I) is known in thorium(I) bromide (ThBr); see Wickleder, Mathias S.; Fourest, Blandine; Dorhout, Peter K. (2006). „Thorium”. Ур.: Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (PDF). 3 (3rd изд.). Dordrecht, the Netherlands: Springer. стр. 52—160. ISBN 978-1-4020-3555-5. doi:10.1007/1-4020-3598-5_3. Архивирано из оригинала (PDF) 7. 3. 2016. г.
- ^ Th(II) and Th(III) are observed in [ThII{η5-C5H3(SiMe3)2}3]− and [ThIII{η5-C5H3(SiMe3)2}3], see Langeslay, Ryan R.; Fieser, Megan E.; Ziller, Joseph W.; Furche, Philip; Evans, William J. (2015). „Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1− anion containing thorium in the formal +2 oxidation state”. Chem. Sci. 6 (1): 517—521. PMC 5811171
 . PMID 29560172. doi:10.1039/C4SC03033H.
. PMID 29560172. doi:10.1039/C4SC03033H.
- ^ U(I) has been observed in uranium monofluoride (UF) and uranium monochloride (UCl), see Sykes, A. G. (1990). „Compounds of Thorium and Uranium”. Advances in Inorganic Chemistry. 34. Academic Press. стр. 87—88. ISBN 978-0-12-023634-3. Приступљено 22. 3. 2015.
- ^ U(II) has been observed in [K(2.2.2-Cryptand)][(C5H4SiMe3)3U], see MacDonald, Matthew R.; Fieser, Megan E.; Bates, Jefferson E.; Ziller, Joseph W.; Furche, Filipp; Evans, William J. (2013). „Identification of the +2 Oxidation State for Uranium in a Crystalline Molecular Complex, [K(2.2.2-Cryptand)][(C5H4SiMe3)3U]”. J. Am. Chem. Soc. 135 (36): 13310—13313. PMID 23984753. doi:10.1021/ja406791t.
- ^ Np(II), (III) and (IV) have been observed, see Dutkiewicz, Michał S.; Apostolidis, Christos; Walter, Olaf; Arnold, Polly L (2017). „Reduction chemistry of neptunium cyclopentadienide complexes: from structure to understanding”. Chem. Sci. 8 (4): 2553—2561. PMC 5431675
 . PMID 28553487. doi:10.1039/C7SC00034K.
. PMID 28553487. doi:10.1039/C7SC00034K.
- ^ Pu(II) has been observed in {Pu[C5H3(SiMe3)2]3}−; see Windorff, Cory J.; Chen, Guo P; Cross, Justin N; Evans, William J.; Furche, Filipp; Gaunt, Andrew J.; Janicke, Michael T.; Kozimor, Stosh A.; Scott, Brian L. (2017). „Identification of the Formal +2 Oxidation State of Plutonium: Synthesis and Characterization ofref name="curium5" {PuII[C5H3(SiMe3)2]3}−”. J. Am. Chem. Soc. 139 (11): 3970—3973. PMID 28235179. doi:10.1021/jacs.7b00706.
- ^ Am(VII) has been observed in AmO5-; see Americium, Das Periodensystem der Elemente für den Schulgebrauch (The periodic table of elements for schools) chemie-master.de (in German), Retrieved 28 November 2010 and Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (II изд.). Oxford: Butterworth-Heinemann. стр. 1265. ISBN 0080379419.
- ^ а б в Cm(V), Bk(V), and Cf(V) have been observed in BkO2+, CfO2+, CmO2(NO3)2−, BkO2(NO3)2−, and CfO2(NO3)2−; see Dau, Phuong Diem; Vasiliu, Monica; Peterson, Kirk A; Dixon, David A; Gibsoon, John K (октобар 2017). „Remarkably High Stability of Late Actinide Dioxide Cations: Extending Chemistry to Pentavalent Berkelium and Californium”. Chemistry - A European Journal. 23 (68): 17369—17378. PMID 29024093. doi:10.1002/chem.201704193.
- ^ а б в Kovács, Attila; Dau, Phuong D.; Marçalo, Joaquim; Gibson, John K. (2018). „Pentavalent Curium, Berkelium, and Californium in Nitrate Complexes: Extending Actinide Chemistry and Oxidation States”. Inorg. Chem. American Chemical Society. 57 (15): 9453—9467. PMID 30040397. doi:10.1021/acs.inorgchem.8b01450.
- ^ Cm(VI) has been observed in curium trioxide (CmO3) and dioxidocurium(2+) (CmO2+); see Domanov, V. P.; Lobanov, Yu. V. (октобар 2011). „Formation of volatile curium(VI) trioxide CmO3”. Radiochemistry. 53 (5): 453—6. doi:10.1134/S1066362211050018.
- ^ Cm(VIII) has been reported to possibly occur in curium tetroxide (CmO4); see Domanov, V. P. (јануар 2013). „Possibility of generation of octavalent curium in the gas phase in the form of volatile tetraoxide CmO4”. Radiochemistry. 55 (1): 46—51. doi:10.1134/S1066362213010098. However, new experiments seem to indicate its nonexistence: Zaitsevskii, Andréi; Schwarz, W H Eugen (април 2014). „Structures and stability of AnO4 isomers, An = Pu, Am, and Cm: a relativistic density functional study”. Physical Chemistry Chemical Physics. 2014 (16): 8997—9001. Bibcode:2014PCCP...16.8997Z. PMID 24695756. doi:10.1039/c4cp00235k.
- ^ Es(IV) is known in einsteinium(IV) fluoride (EsF4); see Kleinschmidt, P (1994). „Thermochemistry of the actinides”. Journal of Alloys and Compounds. 213–214: 169—172. doi:10.1016/0925-8388(94)90898-2.
- ^ Db(V) has been observed in dubnium pentachloride (DbCl5); see H. W. Gäggeler (2007). „Gas Phase Chemistry of Superheavy Elements” (PDF). Paul Scherrer Institute. стр. 26—28. Архивирано из оригинала (PDF) 20. 2. 2012. г.
- ^ Sg(VI) has been observed in seaborgium oxide hydroxide (SgO2(OH)2); see Huebener, S.; Taut, S.; Vahle, A.; Dressler, R.; Eichler, B.; Gäggeler, H. W.; Jost, D.T.; Piguet, D.; et al. (2001). „Physico-chemical characterization of seaborgium as oxide hydroxide” (PDF). Radiochim. Acta. 89 (11–12_2001): 737—741. doi:10.1524/ract.2001.89.11-12.737. Архивирано из оригинала (PDF) 25. 10. 2014. г.
- ^ Sg(0) has been observed in seaborgium hexacarbonyl (Sg(CO)6); see Even, J.; Yakushev, A.; Dullmann, C. E.; Haba, H.; Asai, M.; Sato, T. K.; Brand, H.; Di Nitto, A.; Eichler, R.; Fan, F. L.; Hartmann, W.; Huang, M.; Jager, E.; Kaji, D.; Kanaya, J.; Kaneya, Y.; Khuyagbaatar, J.; Kindler, B.; Kratz, J. V.; Krier, J.; Kudou, Y.; Kurz, N.; Lommel, B.; Miyashita, S.; Morimoto, K.; Morita, K.; Murakami, M.; Nagame, Y.; Nitsche, H.; et al. (2014). „Synthesis and detection of a seaborgium carbonyl complex”. Science. 345 (6203): 1491—3. Bibcode:2014Sci...345.1491E. PMID 25237098. doi:10.1126/science.1255720. (потребна претплата)
- ^ Bh(VII) has been observed in bohrium oxychloride (BhO3Cl); see "Gas chemical investigation of bohrium (Bh, element 107)" Архивирано 2008-02-28 на сајту Wayback Machine, Eichler et al., GSI Annual Report 2000. Retrieved on 2008-02-29
- ^ Hs(VIII) has been observed in hassium tetroxide (HsO4); see „Chemistry of Hassium” (PDF). Gesellschaft für Schwerionenforschung mbH. 2002. Приступљено 31. 1. 2007.
- ^ Cn(II) has been observed in copernicium selenide (CnSe); see „Annual Report 2015: Laboratory of Radiochemistry and Environmental Chemistry” (PDF). Paul Scherrer Institute. 2015. стр. 3.
- ^ Langmuir, Irving (1919). „The arrangement of electrons in atoms and molecules”. J. Am. Chem. Soc. 41 (6): 868—934. doi:10.1021/ja02227a002.